Population pharmacokinetics and optimized dosing of cefuroxime in critically ill patients
- PMID: 40545248
- PMCID: PMC12381626
- DOI: 10.1002/bcp.70144
Population pharmacokinetics and optimized dosing of cefuroxime in critically ill patients
Abstract
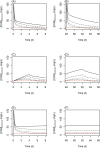
Cefuroxime is a second-generation cephalosporin widely used in the intensive care unit (ICU). ICU patients have high variability in interpatient pharmacokinetics (PK), but the extent of this variation is unclear. We performed an observational PK study in ICU patients. The objective of this study was to gain knowledge on the PK of cefuroxime and investigate target attainment of currently clinically applied dosing regimens. To identify the most suitable regimen the time above the minimal inhibitory concentration of the unbound drug (%fT > MIC) was calculated for different minimal inhibitory concentrations (MICs) and estimated Glomerular Filtration rates (eGFRs). Twenty patients were included with an average age of 66 years and modification of diet in renal disease (MDRD) (not indexed by BSA) of 90 [60-117.5] mL/min. A two-compartment model best fitted the data, with eGFR as a covariate. Probability of target attainment (PTA) was 43% for a 1500-mg q8h bolus dosage for the EUCAST break point of 8 mg/L for a typical individual with a eGFR of 60 mL/min. Dosing continuously using 4.5 g/day obtained 100% PTA for a typical individual with a eGFR up to 120 mL/min.
Keywords: ICU; PK/PD; antibiotics; critical care medicine; intensive care; simulation.
© 2025 The Author(s). British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
All authors report no conflicts of interests.
Figures

References
-
- Pezzani MD. Cefuroxime. In: Kucers' the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. Seventh ed.; 2017.
-
- Garnacho‐Montero J, Garcia‐Garmendia JL, Barrero‐Almodovar A, Jimenez‐Jimenez FJ, Perez‐Paredes C, Ortiz‐Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31(12):2742‐2751. doi: 10.1097/01.CCM.0000098031.24329.10 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

