Cardiovascular Outcomes of Perioperative Sodium-Glucose Transporter 2 Inhibition in Cardiac Surgery Patients: An Open-Label Randomized Pilot Study
- PMID: 40545263
- PMCID: PMC12182971
- DOI: 10.1111/aas.70075
Cardiovascular Outcomes of Perioperative Sodium-Glucose Transporter 2 Inhibition in Cardiac Surgery Patients: An Open-Label Randomized Pilot Study
Abstract
Background: Previous studies have shown cardiovascular benefits of SGLT2 inhibitors. The aim of this study was to evaluate the cardiovascular effects of perioperative SGLT2 inhibition in patients undergoing cardiac surgery.
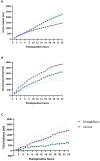
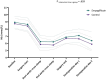
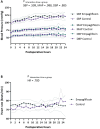
Methods: In this open-label pilot study, adult patients undergoing cardiac surgery were randomized to receive a daily dose of empagliflozin (10 mg; oral) 3 days before surgery until 2 days after surgery, or standard of care. Blood pressure, heart rate, postoperative diuresis, intravenous fluid administration, fluid balance, and vasoactive support were compared between groups during the first 24 postoperative hours.
Results: About 55 patients (sex: 73% male, age: 66 ± 10 years, BMI: 28 ± 4 kg/m2, empagliflozin n = 25, control n = 30) were included in this study and analyzed according to the intention-to-treat principle. Empagliflozin was associated with increased diuresis, mean difference 549 mL (95% CI 258-839, p < 0.001), and less positive fluid balance postoperatively, mean difference -1217 mL (95% CI -2373- -61, p = 0.039). Empagliflozin did not increase the amount of intravenous fluid administered. In the empagliflozin group, norepinephrine was infused for 11.8 ± 11.5 h compared to 19.3 ± 19.3 h in the control group (p = 0.080). No significant between-group differences were observed in postoperative blood pressure and heart rate.
Conclusions: Perioperative SGLT2 inhibition was associated with increased diuresis and lesser fluid accumulation without an increase in vasopressor requirement. These data warrant validation and further evaluation in a larger-scale, double-blind, placebo-controlled trial.
Editorial comment: In this sub-study of the randomized MERCURI-2 trial of perioperative empagliflozin for nondiabetics in cardiac surgery, the authors describe the hemodynamic outcomes and fluid status of the patients. The authors noted a higher urine output and a more negative fluid balance in the intervention group compared to the placebo group. An interesting observation is the trend towards lower noradrenaline usage, although this cannot be concluded with confidence based on this data. The findings support considering and further studying the use of these medications for patients with cardiovascular disease undergoing surgery.
Trial registration: https://onderzoekmetmensen.nl/en/trial/26563 Identifier: NL9561.
© 2025 The Author(s). Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation.
Conflict of interest statement
D.H.v.R. has served as a consultant and received honoraria from Boehringer Ingelheim and Lilly, Merck, Sanofi, and AstraZeneca and has received research operating funds from Boehringer Ingelheim and Lilly Diabetes Alliance and AstraZeneca; all honoraria are paid to his employer (Amsterdam University Medical Center, Amsterdam). The other authors declare no conflicts of interest.
Figures

References
-
- Packer M., Anker S. D., Butler J., et al., “Cardiovascular and Renal Outcomes With Empagliflozin in Heart Failure,” New England Journal of Medicine 383, no. 15 (2020): 1413–1424. - PubMed
-
- McMurray J. J. V., Solomon S. D., Inzucchi S. E., et al., “Dapagliflozin in Patients With Heart Failure and Reduced Ejection Fraction,” New England Journal of Medicine 381, no. 21 (2019): 1995–2008. - PubMed
-
- Usman M. S., Bhatt D. L., Hameed I., et al., “Effect of SGLT2 Inhibitors on Heart Failure Outcomes and Cardiovascular Death Across the Cardiometabolic Disease Spectrum: A Systematic Review and Meta‐Analysis,” Lancet Diabetes and Endocrinology 12, no. 7 (2024): 447–461. - PubMed
-
- Anker S. D., Butler J., Filippatos G., et al., “Empagliflozin in Heart Failure With a Preserved Ejection Fraction,” New England Journal of Medicine 385, no. 16 (2021): 1451–1461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

