Evaluation of cognitive, functional, and behavioral effects observed in EMERGE, a phase 3 trial of aducanumab in people with early Alzheimer's disease
- PMID: 40545559
- PMCID: PMC12183105
- DOI: 10.1002/alz.70224
Evaluation of cognitive, functional, and behavioral effects observed in EMERGE, a phase 3 trial of aducanumab in people with early Alzheimer's disease
Abstract
Introduction: In EMERGE (NCT02484547), participants receiving aducanumab had significantly less progression versus placebo on all prespecified clinical endpoints at week 78. Here, we explicate the clinical meaningfulness of these treatment effects by analyzing item-level data and the persistence of treatment benefit.
Methods: Participants with early Alzheimer's disease (AD) were stratified by apolipoprotein E (APOE) ε4 status and randomized (1:1:1) to receive low- or high-dose aducanumab, or placebo. Prespecified principal component analyses (PCAs) per the Statistical Analysis Plan were followed by post hoc examination of individual domains/items across all five clinical endpoints. Progression analysis assessed reduction in clinical decline.
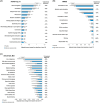
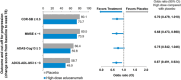
Results: High-dose aducanumab demonstrated clinically meaningful slowing of progression across clinical endpoints measuring cognition, daily function, and behavioral symptoms. Delay of progression over 18 months was consistent across measures; treatment effects increased over time.
Discussion: Across multiple analyses aducanumab slowed cognitive decline, prolonged functional independence, and attenuated behavioral symptoms in participants with early AD. These outcomes comprise the elements of a clinically meaningful response to treatment.
Highlights: Endpoints in EMERGE assessed different aspects of cognition, daily function, and behavioral symptoms. Treatment benefits were observed across subdomains on all five clinical endpoints. Aducanumab meaningfully slowed disease progression in participants with early AD.
Keywords: Alzheimer's disease; anti‐Aβ mAb; behavioral symptoms; clinical meaningfulness; cognitive decline; functional independence; treatment effect.
© 2025 Biogen. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
J.M., H.M.B., M.N., J.O., and F.F. are employees and shareholders of Biogen. P.H., M.P., C.M., Y.T., L.Y., and S.B.H. were employees of Biogen at the time of this study and may own stock in Biogen. J.C. has provided consultation to Acadia, Acumen, ALZpath, Aprinoia, Artery, Biogen, Biohaven, BioXcel, Bristol Myers Squib, Eisai, Fosun, GAP Foundation, Janssen, Karuna, Kinoxis, Lighthouse, Lilly, Lundbeck, LSP/EQT, Merck, MoCA Cognition, New Amsterdam, Novo Nordisk, Optoceutics, Otsuka, Oxford Brain Diagnostics, Praxis, Prothena, ReMYND, Roche, Scottish Brain Sciences, Signant Health, Simcere, Sinaptica, TrueBinding, and Vaxxinity pharmaceutical, assessment, and investment companies. J.C. owns the copyright of the Neuropsychiatric Inventory. J.C. has stocks/options in Artery, Vaxxinity, Behrens, Alzheon, MedAvante‐Prophase, and Acumen. J.C. is supported by NIGMS grant P20GM109025; NIA grant R35AG71476; NIA R25 AG083721‐01; ADDF; Ted and Maria Quirk Endowment; and Joy Chambers‐Grundy Endowment. S.C. was an ENGAGE trial site investigator and an Aducanumab Steering Committee member. She is a consultant (no personal fees) to Alnylam, Biogen, Bristol Myers Squibb, Cassava Sciences, Cognivue, Cogstate, Eisai, Eli Lilly, INmune Bio, Lundbeck, Novartis, Novo Nordisk, Parexel, RetiSpec, Roche, and SciNeuro and receives research support (paid to institution) from AbbVie, Alnylam, AgeneBio, Alector, Alzheon, Anavex, Biogen, Cassava Sciences, Eisai, Eli Lilly, GSK, INmune Bio, Janssen, Novo Nordisk, RetiSpec, Roche, and UCB Biopharma. J.H. is an employee and shareholder in Scottish Brain Sciences. Additionally, he holds stock options in Neurotrack Inc. and is a joint holder of patents with MyCognition Ltd. J.J. is the owner and President of CognitionMetrics, L.L.C., which received fees from Biogen in consideration of scientific consulting services. C.J.M. was an ENGAGE trial site investigator and an Aducanumab Steering Committee member. She is supported by NIHR Biomedical Research Centre at UCLH and has acted as a consultant to Biogen, Eli Lilly, Roche, IONIS, Alector, Eisai, Alnylam, and Novartis. She has received research support from Biogen. A.P. reports personal fees from Acadia Pharmaceuticals, Avanir, Cadent Therapeutics, Functional Neuromodulation, Syneos, and BioXcel and grants to his institution from Avanir, Biogen, Biohaven, Eisai, Eli Lilly, Genentech/Roche, and Novartis. Author disclosures are available in the supporting information.
Figures



References
-
- Aduhelm (aducanumab‐avwa) . Prescribing information. Biogen, Inc; 2021. Accessed [July 27, 2021].
-
- Leber P, Guidelines for the Clinical Evaluation of antiDementia Drugs (1990 draft). 1990.
-
- US Food and Drug Administration . Early Alzheimer's Disease: Developing Drugs for Treatment Guidance for Industry. Guidance Compliance Regulatory Information. 2024.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

