Programmed death-ligand 1 expression and prognostic significance in bevacizumab treated ovarian cancer patients: Results from the phase IV MITO16A/MaNGO OV-2 translational study
- PMID: 40545725
- PMCID: PMC12183328
- DOI: 10.1002/ctm2.70373
Programmed death-ligand 1 expression and prognostic significance in bevacizumab treated ovarian cancer patients: Results from the phase IV MITO16A/MaNGO OV-2 translational study
Conflict of interest statement
The authors declare no conflict of interest.
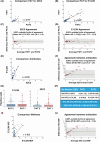
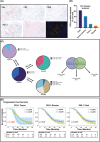
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
