Cell-Surface LAMP1 is a Senescence Marker in Aging and Idiopathic Pulmonary Fibrosis
- PMID: 40545776
- PMCID: PMC12419843
- DOI: 10.1111/acel.70141
Cell-Surface LAMP1 is a Senescence Marker in Aging and Idiopathic Pulmonary Fibrosis
Abstract
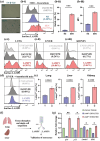
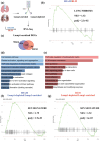
The accumulation of senescent cells (SEN) with aging produces a chronic inflammatory state that accelerates age-related diseases. Eliminating SEN has been shown to delay, prevent, and in some cases reverse aging in animal disease models and extend lifespan. There is thus an unmet clinical need to identify and target SEN while sparing healthy cells. Here, we show that Lysosomal-Associated Membrane Protein 1 (LAMP1) is a membrane-specific biomarker of cellular senescence. We have validated selective LAMP1 upregulation in SEN in human and mouse cells. Lamp1+ cells express high levels of the prototypical senescence markers p16, p21, Glb1, and have low Lmnb1 expression as compared to Lamp1- cells. The percentage of Lamp1+ cells is increased with age and in mice with fibrotic lungs due to bleomycin (BLM) instillation. The RNA-Sequencing analysis of the Lamp1-enriched populations in sham and BLM mice lung tissue revealed enrichment of several senescence-related genes in both groups when compared to the SenMayo gene set derived from transcriptomic profiling of senescence markers in Mayo Clinic research datasets. Finally, we use a dual antibody-drug conjugate (ADC) strategy to eliminate SEN in cell culture assay.
Keywords: ADC; LAMP1; aging; biomarker; senescence; surface.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
A patent application related to the work described in this manuscript has been filed by the SENS Research Foundation (SRF), now operating as the Lifespan Research Institute (LRI), with A.S. listed as an inventor. All authors are or have been employees of SRF or LRI and have received salary support from these organizations. The authors declare no conflicts of interest.
Figures




References
-
- Agarwal, A. K. , Srinivasan N., Godbole R., et al. 2015. “Role of Tumor Cell Surface Lysosome‐Associated Membrane Protein‐1 (LAMP1) and Its Associated Carbohydrates in Lung Metastasis.” Journal of Cancer Research and Clinical Oncology 141, no. 9: 1563–1574. 10.1007/s00432-015-1917-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

