Impact of Obesity on Treatment Response in Patients With Chronic Inflammatory Disease Receiving Biologic Therapy: Secondary Analysis of the Prospective Multicentre BELIEVE Cohort Study
- PMID: 40545788
- PMCID: PMC12183491
- DOI: 10.1111/sji.70035
Impact of Obesity on Treatment Response in Patients With Chronic Inflammatory Disease Receiving Biologic Therapy: Secondary Analysis of the Prospective Multicentre BELIEVE Cohort Study
Abstract
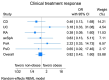
Biological therapy is used to treat chronic inflammatory diseases (CIDs); however, up to 50% of patients fail to achieve an adequate clinical response. This study aimed to access the impact of obesity on clinical treatment response in CID patients after 14-16 weeks of biological therapy. This multicentre prospective cohort study enrolled 233 adults between 2017 and 2020 diagnosed with Crohn disease, ulcerative colitis (UC), rheumatoid arthritis, axial spondyloarthritis (PsA), psoriatic arthritis or psoriasis scheduled for biologic therapy. The main analysis population included patients with BMI data before treatment initiation, categorising patients as either obese (BMI ≥ 30 kg/m2) or non-obese (BMI < 30 kg/m2). The primary endpoint was the proportion of patients achieving clinical treatment response after 14-16 weeks. Main analyses were based on logistic regression with a factor for obesity, while adjusted for sex and age. Of the 228 patients eligible for the main analyses, 125 (55%) responded to biologic therapy. In the obese group (n = 59), 30 (51%) patients responded compared to the 95 (56%) individuals categorised as non-obese (n = 169), with no difference between groups (OR: 0.82, 95% CI: 0.43 to 1.60). This study did not find a lower likelihood of response to biologics in obese individuals compared with non-obese counterparts. Trial Registration: ClinicalTrials.gov identifier: NCT03173144.
Keywords: Crohn disease; autoimmune disease; axial spondyloarthritis; biological therapy; body mass index; psoriasis; psoriatic arthritis; rheumatoid arthritis; treatment outcome; ulcerative colitis.
© 2025 The Author(s). Scandinavian Journal of Immunology published by John Wiley & Sons Ltd on behalf of The Scandinavian Foundation for Immunology.
Conflict of interest statement
V.A. has served as advisory board member for MSD (Merck). J.B.B. has served as a consultant for Janssen‐Cilag A/S, and support for attending meetings with Pfiser and Tillots. R.C. has served as a consultant for IAG and Compass Communications Ltd. R.C. has served as statistical editor for Osteoarthritis and Cartilage. R.C. has served as statistical editor for Acta Orthopaedica. The other authors declare no conflicts of interest.
Figures

References
-
- Molodecky N. A., Soon I. S., Rabi D. M., et al., “Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review,” Gastroenterology 142 (2012): 46–54. - PubMed
-
- Cao F., He Y. S., Wang Y., et al., “Global Burden and Cross‐Country Inequalities in Autoimmune Diseases From 1990 to 2019,” Autoimmunity Reviews 22 (2023): 103326. - PubMed
-
- Cross M., Smith E., Hoy D., et al., “The Global Burden of Rheumatoid Arthritis: Estimates From the Global Burden of Disease 2010 Study,” Annals of the Rheumatic Diseases 73 (2014): 1316–1322. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

