A Microfluidic Device to Realize Electrochemically Controlled SERS Detection in HPLC
- PMID: 40545866
- PMCID: PMC12224159
- DOI: 10.1021/acs.analchem.5c02232
A Microfluidic Device to Realize Electrochemically Controlled SERS Detection in HPLC
Abstract
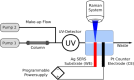
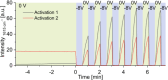
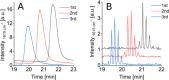
Surface-enhanced Raman spectroscopy (SERS) is a powerful technique for vibrational spectroscopy, but analyzing mixtures in solution remains challenging due to spectral overlap. Integrating SERS with a separation method, such as high-performance liquid chromatography (HPLC), offers a promising solution. However, online coupling has been limited by the compatibility issues between the SERS process and flow-based systems, which can result in either irreversible analyte adsorption on the SERS substrate or insufficient interaction. This can lead to signal carry-over or low sensitivity. In this study, we present the first HPLC-compatible, pressure-stable SERS flow cell designed for real-time analysis under continuous flow. Fabricated entirely from glass using selective laser etching, the monolithic flow cell incorporates a silver-based SERS substrate and a counter electrode, enabling online electrochemical SERS (EC-SERS) experiments. Electrochemical control facilitates on-demand substrate activation, thereby enhancing signal intensity, extending substrate lifetime, and eliminating memory effects. This approach broadens the range of detectable analytes, including those that are traditionally difficult to detect using passive SERS. We demonstrate the performance of the system through HPLC-SERS analyses of model dyes (e.g., crystal violet, malachite green, and rhodamine) and pharmaceutical compounds (e.g., cyanocobalamin and folic acid). This innovation introduces a novel SERS-based HPLC detection method, supporting the seamless integration of SERS into high-throughput analytical workflows.
Figures



References
-
- Han X. X., Rodriguez R. S., Haynes C. L., Ozaki Y., Zhao B.. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers. 2021;1:87. doi: 10.1038/s43586-021-00083-6. - DOI
-
- Li C., Huang Y., Li X., Zhang Y., Chen Q., Ye Z., Alqarni Z., Bell S. E. J., Xu Y.. J. Mater. Chem. C. 2021;9:11517. doi: 10.1039/D1TC02134F. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

