Antitumoral Efficacy of AuNRs-Laden ECFCs In Vitro and In Vivo: Decoding the Heat and Rays Combo Treatment in Breast Cancer and Melanoma Cells
- PMID: 40545912
- PMCID: PMC12391628
- DOI: 10.1002/adhm.202502416
Antitumoral Efficacy of AuNRs-Laden ECFCs In Vitro and In Vivo: Decoding the Heat and Rays Combo Treatment in Breast Cancer and Melanoma Cells
Abstract
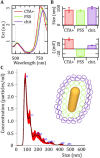
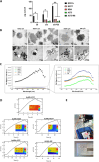
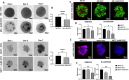
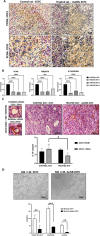
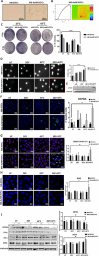
Radiotherapy remains a cornerstone in metastatic cancer treatment but is often hindered by tumor hypoxia and radioresistance. Gold nanorods (AuNRs) offer promise in enhancing radiotherapy through hyperthermia, yet their clinical impact is limited by poor tumor targeting. Building on the previous findings demonstrating the tumor-homing ability of Endothelial Colony Forming Cells (ECFCs) loaded with AuNRs, this study advances their use as a biologically targeted delivery system for precise radiotherapy enhancement. Using 3D in vitro tumor models and in vivo studies with nude rats, it is demonstrated that ECFCs actively home to hypoxic tumor regions, overcoming traditional nanoparticle delivery limitations. This targeted approach ensures efficient AuNR accumulation, enhancing photothermal activation and maximizing radiosensitization. In vitro, ECFC-loaded AuNRs significantly amplify radiotherapy effects, inducing ferroptosis in melanoma and inhibiting autophagy in breast cancer cells-revealing distinct tumor-specific mechanisms. Moreover, ECFC-AuNRs suppress tumor proliferation and angiogenesis, blocking vessel-like structure formation in vitro and in vivo. By integrating cellular therapy with nanotechnology, this study presents a novel strategy to counter radioresistance and improve therapeutic precision. These findings lay the foundation for a clinically viable, patient-specific approach, unlocking new possibilities in advanced cancer treatment.
Keywords: Endothelial Colony Forming Cells (ECFCs); autophagy; breast cancer; ferroptosis; gold nanorods; hyperthermia; melanoma; radiotherapy.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

