Bacterial Extracellular Vesicles Exploit Filopodial Surfing and Retraction Mechanisms to Reach the Host Cell Body in an Actin-Dependent Manner
- PMID: 40545962
- PMCID: PMC12183400
- DOI: 10.1002/jev2.70107
Bacterial Extracellular Vesicles Exploit Filopodial Surfing and Retraction Mechanisms to Reach the Host Cell Body in an Actin-Dependent Manner
Abstract
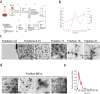
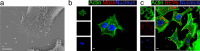
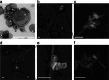
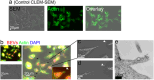
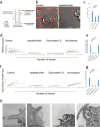
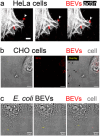
Extracellular vesicles derived from gram-negative bacteria are nano-sized particles of different size and origin released by these microbes and are collectively called bacterial extracellular vesicles (BEVs). These BEVs may serve as vehicles for delivering bacterial molecules to eukaryotic host cells. Depending on the bacterial species, BEVs elicit various host cellular and immunomodulatory responses, often aiding bacterial survival and communication. Early events in the initial interaction between BEVs and the host cell, as well as how BEVs reach the cell body, remain unexplored. In this study, we describe the interaction of BEVs with actin-rich cellular extensions, including filopodia and retraction fibres, which extend from the host cell surface. Using microscopy-based tracking at the single cell level, BEVs were shown to exploit cellular extensions at the cell periphery to reach the main cell body, either by hijacking retracted extensions or by surfing along these extensions in an actin-dependent manner. BEVs bind to the outer surface of the extensions, but no internalization occurs at this stage. Instead, they serve as transport for BEVs to the main cell body, where endocytosis takes place. Importantly, this process appears to be a general phenomenon for BEVs across different bacterial species and cell origins.
Keywords: bacterial extracellular vesicles (BEVs); extracellular processing; filopodia and cellular extensions.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Ayala, G. , Torres L., Espinosa M., Fierros‐Zarate G., Maldonado V., and Meléndez‐Zajgla J.. 2006. “External Membrane Vesicles From Helicobacter Pylori Induce Apoptosis in Gastric Epithelial Cells: External Membrane Vesicles From H. Pylori Induce Apoptosis.” FEMS Microbiology Letters 260: 178–185. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

