Macrophages Internalize Epithelial-Derived Extracellular Vesicles That Contain Ferritin via the Macrophage Scavenger Receptor 1 to Promote Inflammatory Bowel Disease
- PMID: 40545965
- PMCID: PMC12183398
- DOI: 10.1002/jev2.70105
Macrophages Internalize Epithelial-Derived Extracellular Vesicles That Contain Ferritin via the Macrophage Scavenger Receptor 1 to Promote Inflammatory Bowel Disease
Abstract
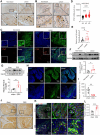
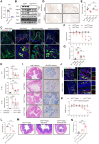
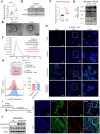
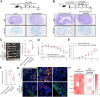
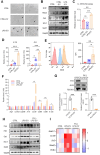
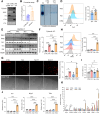
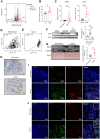
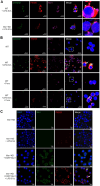
The incidence of inflammatory bowel disease (IBD) is on the rise, yet current clinical treatments are limited. Previous studies have identified impairments in both systemic and local iron metabolism in IBD patients. However, the impact of iron dyshomeostasis on the development and pathogenesis of IBD remains elusive. In this study, we confirmed iron deposition in inflamed intestinal lesions of IBD patients and mice with DSS-induced colitis, accompanied by distinct distribution patterns of the iron storage protein ferritin. To reveal the role of ferritin in the involvement of pathology of IBD, we constructed intestinal epithelial cell- or myeloid-specific ferritin H (FtH) knockout mice and demonstrated that intestinal epithelial cells (IECs) release extracellular vesicles (EVs) that contain iron-loaded ferritin. These EVs are internalized by macrophages via the macrophage scavenger receptor 1 (Msr1), leading to the activation of inflammatory responses and oxidative stress, thereby exacerbating colitis severity. Genetic deletion of FtH in IECs or blockage of macrophage ferritin uptake, either through Msr1 inhibitor fucoidan or through Msr1 knockdown (KD), suppressed inflammatory symptoms. Thus, EVs containing iron-loaded ferritin released from IECs activate macrophages and contribute to IBD development, supporting that IBD patients with iron deficiency anaemia are often prescribed iron supplementation in a remission phase, other than in an active phase of the disease. Pharmacological inhibition of this ferritin secretion and engulfing process provides a therapeutic target for the disease.
Keywords: IBD; extracellular vesicles; ferritin trafficking; intestinal epithelial cells; macrophage scavenger receptor 1; macrophages.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures
Similar articles
-
Pannexin-1 Aggravates Inflammatory Bowel Disease via Unbalancing Macrophage Polarisation and Triggering Ferroptosis in Mice.Immunology. 2025 Sep;176(1):116-131. doi: 10.1111/imm.13948. Epub 2025 Jun 9. Immunology. 2025. PMID: 40488292
-
Odoribacter splanchnicus-derived extracellular vesicles alleviate inflammatory bowel disease by modulating gastrointestinal inflammation and intestinal barrier function via the NLRP3 inflammasome suppression.Mol Med. 2025 Feb 11;31(1):56. doi: 10.1186/s10020-025-01063-2. Mol Med. 2025. PMID: 39934686 Free PMC article.
-
Bone marrow mesenchymal stem cell-derived exosomes alleviate DSS-induced inflammatory bowel disease in mice through inhibiting intestinal epithelial cell pyroptosis via delivery of TSG-6.Front Immunol. 2025 Jun 30;16:1601591. doi: 10.3389/fimmu.2025.1601591. eCollection 2025. Front Immunol. 2025. PMID: 40661945 Free PMC article.
-
The Relationship Between CSF1R Signaling, Monocyte-Macrophage Differentiation, and Susceptibility to Inflammatory Bowel Disease.Cell Mol Gastroenterol Hepatol. 2025;19(8):101510. doi: 10.1016/j.jcmgh.2025.101510. Epub 2025 Mar 26. Cell Mol Gastroenterol Hepatol. 2025. PMID: 40154882 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Arosio, P. , Ingrassia R., and Cavadini P.. 2009. “Ferritins: a family of Molecules for Iron Storage, Antioxidation and More.” Biochimica Et Biophysica Acta 1790, no. 7: 589–599. - PubMed
-
- Carrier, J. , Aghdassi E., Cullen J., and Allard J. P.. 2002. “Iron Supplementation Increases Disease Activity and Vitamin E Ameliorates the Effect in Rats With Dextran Sulfate Sodium‐Induced Colitis.” Journal of Nutrition 132, no. 10: 3146–3150. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

