Metabolically Engineered Extracellular Vesicles Released From a Composite Hydrogel Delivery System Regulate the Microenvironment for Periprosthetic Osteolysis Treatment
- PMID: 40545972
- PMCID: PMC12183387
- DOI: 10.1002/jev2.70098
Metabolically Engineered Extracellular Vesicles Released From a Composite Hydrogel Delivery System Regulate the Microenvironment for Periprosthetic Osteolysis Treatment
Abstract
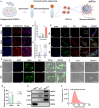
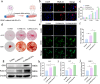
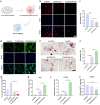
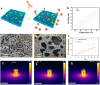
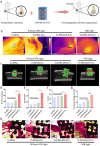
Despite remarkable progress in total joint arthroplasty, aseptic loosening of titanium (Ti) alloy persists as a critical clinical challenge due to the poor wear resistance and biological inertness of such implants. Targeting of inflammatory osteolysis and remodelling of the osseointegration environment represent promising therapeutic approaches to address this issue. In this study, we developed a novel engineered extracellular vesicles (EVs) with a tag of dextran sulfate (DS-EVs) via metabolic glycan labelling (MGL)-mediated click chemistry. This targeted delivery of EVs, derived from metabolically engineered stem cells, establishes a new cell-free therapeutic system for periprosthetic osteolysis. DS-EVs demonstrated specific macrophage tropism, effectively reprogramming macrophage polarisation from pro-inflammatory M1 to regenerative M2 phenotypes. This phenotypic shift attenuated osteoclastogenesis while enhancing osseointegration through GPC6/Wnt pathway activation in vitro. Furthermore, we designed a multifunctional 3D titanium alloy scaffold with MXene-PVA composite hydrogel coatings (Ti-PPM scaffold). The multifunctional Ti-PPM composite scaffold, incorporating DS-EVs, provides a robust delivery system for periprosthetic osteolysis. This integrated system exhibits dual advantages of enhanced wear resistance and optimised interfacial adhesion, while enabling controlled EV release to maximize DS-EVs' osseointegration potential in vivo. Collectively, our findings establish DS-EVs as a transformative therapeutic modality for periprosthetic osteolysis through dual modulation of the osseointegration microenvironment and macrophage phenotypic heterogeneity.
Keywords: extracellular vesicles; hydrogel; macrophage; periprosthetic osteolysis; titanium alloys.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mangiferin- and GNPs/ECPP-loaded platform of UH with dual bi-directional dynamic modulation of stem cells/macrophages and osteoblasts/osteoclasts for the prevention of aseptic loosening.J Mater Chem B. 2025 Jan 2;13(2):695-710. doi: 10.1039/d4tb02079k. J Mater Chem B. 2025. PMID: 39620621
-
Erythropoietin-Stimulated Macrophage-Derived Extracellular Vesicles in Chitosan Hydrogel Rescue BMSCs Fate by Targeting EGFR to Alleviate Inflammatory Bone Loss in Periodontitis.Adv Sci (Weinh). 2025 Jun;12(23):e2500554. doi: 10.1002/advs.202500554. Epub 2025 Apr 28. Adv Sci (Weinh). 2025. PMID: 40289904 Free PMC article.
-
Nanozyme Coating-Mediated Mitochondrial Metabolic Reprogramming of Macrophages for Immunomodulatory Osseointegration in Rheumatoid Arthritis Case.ACS Nano. 2025 Jul 22;19(28):26127-26146. doi: 10.1021/acsnano.5c07535. Epub 2025 Jul 8. ACS Nano. 2025. PMID: 40628648
-
Extracellular Vesicle-Integrated Biomaterials in Bone Tissue Engineering Applications: Current Progress and Future Perspectives.Int J Nanomedicine. 2025 Jun 17;20:7653-7683. doi: 10.2147/IJN.S522198. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40546799 Free PMC article. Review.
-
Extracellular vesicles: the "Trojan Horse" within breast cancer host microenvironments.Mol Cancer. 2025 Jun 23;24(1):183. doi: 10.1186/s12943-025-02358-y. Mol Cancer. 2025. PMID: 40551109 Free PMC article. Review.
References
-
- Abd‐Elaziem, W. , Darwish M. A., Hamada A., and Daoush W. M.. 2024. “Titanium‐Based Alloys and Composites for Orthopedic Implants Applications: A Comprehensive Review.” Materials & Design 241: 112850.
-
- Adams, C. T. , O'Connor C. M., Young J. R., Anoushiravani A. A., Doherty B. S., and Congiusta F.. 2021. “Outcomes of a Total Joint Arthroplasty Enhanced Recovery Program in a Community Hospital Setting.” Journal of Arthroplasty 36, no. 7: S173–S178. - PubMed
-
- An, J. X. , Han Z. Y., Qin Y. T., Li C. X., He J. L., and Zhang X. Z.. 2024. “Bacteria‐Based Backpacks to Enhance Adoptive Macrophage Transfer Against Solid Tumors.” Advanced Materials 36, no. 6: e2305384. - PubMed
MeSH terms
Substances
Grants and funding
- 22YF1447500/Shanghai "Science and Technology Innovation Action Plan" Morning Star Cultivation
- ChenGuang project
- 82372388/National Natural Science Foundation of China
- 2023PY20/Basic Medical Research Foundation of Naval Medical University
- 2023MS022/Basic Medical Research Foundation of Naval Medical University
LinkOut - more resources
Full Text Sources
Miscellaneous

