Metabolically Engineered Extracellular Vesicles Released From a Composite Hydrogel Delivery System Regulate the Microenvironment for Periprosthetic Osteolysis Treatment
- PMID: 40545972
- PMCID: PMC12183387
- DOI: 10.1002/jev2.70098
Metabolically Engineered Extracellular Vesicles Released From a Composite Hydrogel Delivery System Regulate the Microenvironment for Periprosthetic Osteolysis Treatment
Abstract
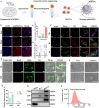
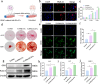
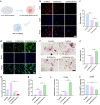
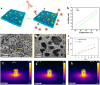
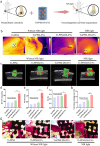
Despite remarkable progress in total joint arthroplasty, aseptic loosening of titanium (Ti) alloy persists as a critical clinical challenge due to the poor wear resistance and biological inertness of such implants. Targeting of inflammatory osteolysis and remodelling of the osseointegration environment represent promising therapeutic approaches to address this issue. In this study, we developed a novel engineered extracellular vesicles (EVs) with a tag of dextran sulfate (DS-EVs) via metabolic glycan labelling (MGL)-mediated click chemistry. This targeted delivery of EVs, derived from metabolically engineered stem cells, establishes a new cell-free therapeutic system for periprosthetic osteolysis. DS-EVs demonstrated specific macrophage tropism, effectively reprogramming macrophage polarisation from pro-inflammatory M1 to regenerative M2 phenotypes. This phenotypic shift attenuated osteoclastogenesis while enhancing osseointegration through GPC6/Wnt pathway activation in vitro. Furthermore, we designed a multifunctional 3D titanium alloy scaffold with MXene-PVA composite hydrogel coatings (Ti-PPM scaffold). The multifunctional Ti-PPM composite scaffold, incorporating DS-EVs, provides a robust delivery system for periprosthetic osteolysis. This integrated system exhibits dual advantages of enhanced wear resistance and optimised interfacial adhesion, while enabling controlled EV release to maximize DS-EVs' osseointegration potential in vivo. Collectively, our findings establish DS-EVs as a transformative therapeutic modality for periprosthetic osteolysis through dual modulation of the osseointegration microenvironment and macrophage phenotypic heterogeneity.
Keywords: extracellular vesicles; hydrogel; macrophage; periprosthetic osteolysis; titanium alloys.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Abd‐Elaziem, W. , Darwish M. A., Hamada A., and Daoush W. M.. 2024. “Titanium‐Based Alloys and Composites for Orthopedic Implants Applications: A Comprehensive Review.” Materials & Design 241: 112850.
-
- Adams, C. T. , O'Connor C. M., Young J. R., Anoushiravani A. A., Doherty B. S., and Congiusta F.. 2021. “Outcomes of a Total Joint Arthroplasty Enhanced Recovery Program in a Community Hospital Setting.” Journal of Arthroplasty 36, no. 7: S173–S178. - PubMed
-
- An, J. X. , Han Z. Y., Qin Y. T., Li C. X., He J. L., and Zhang X. Z.. 2024. “Bacteria‐Based Backpacks to Enhance Adoptive Macrophage Transfer Against Solid Tumors.” Advanced Materials 36, no. 6: e2305384. - PubMed
MeSH terms
Substances
Grants and funding
- 22YF1447500/Shanghai "Science and Technology Innovation Action Plan" Morning Star Cultivation
- ChenGuang project
- 82372388/National Natural Science Foundation of China
- 2023PY20/Basic Medical Research Foundation of Naval Medical University
- 2023MS022/Basic Medical Research Foundation of Naval Medical University
LinkOut - more resources
Full Text Sources
Miscellaneous

