Anticancer Potential of Myricetin against Huh7- and Hep3B-Derived Liver Cancer Stem Cells through the Regulation of Apoptosis, Autophagy, and Stemness
- PMID: 40545984
- PMCID: PMC12215034
- DOI: 10.4062/biomolther.2025.044
Anticancer Potential of Myricetin against Huh7- and Hep3B-Derived Liver Cancer Stem Cells through the Regulation of Apoptosis, Autophagy, and Stemness
Abstract
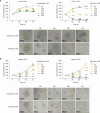
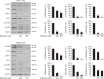
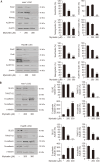
Liver cancer stem cells (LCSCs) play a significant role in the development, metastasis, treatment resistance, and recurrence of hepatocellular carcinoma (HCC). Targeting LCSCs offers a novel strategy to overcome treatment resistance in HCC. Myricetin, a flavonol from the flavonoid family, is known for its diverse biological activities, including anticancer effects. However, its potential for eradicating LCSCs had not been thoroughly investigated prior to this study. This study evaluated the effects of myricetin on LCSCs derived from Huh7 and Hep3B cell lines both in vitro and in vivo. LCSCs were treated with myricetin to assess cell proliferation, cell cycle arrest, apoptosis induction, autophagy regulation, stemness and EMT marker expression, and tumor growth suppression using a chicken embryo CAM model. Additionally, the combination therapy of myricetin with chloroquine, an autophagy inhibitor, was explored. Myricetin significantly inhibited the proliferation of Huh7- and Hep3B-derived LCSCs and suppressed tumor growth in the CAM model. It induced cell cycle arrest at the G0/G1 phase and triggered apoptosis through intrinsic and extrinsic pathways. Myricetin also stimulated autophagy by inhibiting the PI3K/AKT/mTOR pathway, reduced the expression of stemness markers, including Sox2, Oct4, Nanog, and ALDH1A1, and suppressed EMT. Combining myricetin with chloroquine enhanced apoptotic effects and further downregulated stemness markers by inhibiting STAT3 activation, demonstrating greater efficacy than myricetin alone. The findings establish myricetin, either as a standalone treatment or in combination with chloroquine, as a promising therapeutic candidate for targeting LCSC growth and overcoming chemotherapy resistance in HCC.
Keywords: Apoptosis; Autophagy; Chloroquine; Liver cancer stem cell; Myricetin.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Figures
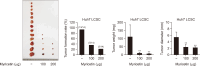





References
-
- Аrtykov А. А., Belov D. A., Shipunova V. O., Trushina D. B., Deyev S. M., Dolgikh D. A., Kirpichnikov M. P., Gasparian M. E. Chemotherapeutic agents sensitize resistant cancer cells to the DR5-specific variant DR5-B more efficiently than to TRAIL by modulating the surface expression of death and decoy receptors. Cancers. 2020;12:1129. doi: 10.3390/cancers12051129. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

