Pain Experiences and Prescription Pain Medications Among People With Selected Muscular Dystrophies in the Muscular Dystrophy Surveillance, Tracking, and Research Network
- PMID: 40546227
- PMCID: PMC12306532
- DOI: 10.1002/mus.28460
Pain Experiences and Prescription Pain Medications Among People With Selected Muscular Dystrophies in the Muscular Dystrophy Surveillance, Tracking, and Research Network
Abstract
Introduction/aims: Pain is a recognized symptom of muscular dystrophy (MD), but little is known about prescription pain medications in this population. We describe pain experiences and pain medications prescribed for individuals with selected MDs using population-based surveillance data collected by the Muscular Dystrophy Surveillance, Tracking, and Research Network.
Methods: Pain and prescription data were abstracted from medical records for 1282 individuals with Duchenne and Becker (DBMD) MD during 2000-2015 and congenital (CMD), distal (DD), Emery-Dreifuss (EDMD), facioscapulohumeral (FSHD), limb-girdle (LGMD), and myotonic (DM) MDs during 2008-2016. Percentages of individuals prescribed pain medications for ≥ 6 weeks during follow-up were estimated. Logistic regression was used to examine associations with selected demographic and clinical characteristics.
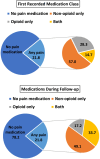
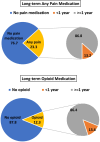
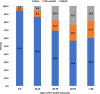
Results: Moderate pain was observed among 34% of all people with available pain scores and varied by MD type (13%-53%). Pain medications were prescribed for 31.1%-40.2% of people 20 years and older, but less frequently (< 15%) among people less than 20 years old. Among people prescribed pain medications, the first medication was typically a non-opioid (57%), but both non-opioid and opioid medication classes were prescribed during follow-up (34%). Pain medications were typically prescribed for longer than 1 year (> 85%). Impaired mobility had the strongest association with prescription pain medication.
Discussion: The prescription of pain medication is common for people with symptomatic MD. Most people were prescribed only non-opioids. These data highlight pain management as a frequent component of MD care. Understanding modifiable factors associated with MD-related pain and effective interventions may help improve care.
Keywords: facioscapulohumeral muscular dystrophy; limb‐girdle muscular dystrophy; muscular dystrophy; myotonic dystrophy; prescription pain medication.
© 2025 The Author(s). Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
K.D.M. receives research funding from the Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant (NIH U54 NS053672), and the Centers for Disease Control (U01 DD001248). She serves as an advisory board member for MDA and the FSH Society; is a board member for the Friedreich Ataxia Research Alliance (FARA); receives or has recently received clinical trial funding from PTC Therapeutics, Sarepta Therapeutics, Pfizer, Reata, Italfarmaco, Fibrogen, Italfarmaco, CSL Behring, AMO and Reata. S.A.R. serves on scientific advisory committees for pregnancy registries for Harmony Biosciences, Axsome Therapeutics, Biohaven Pharmaceuticals (recently acquired by Pfizer), and Myovant Sciences. N.E.J. has received grant funding from NINDS (R01NS104010, U01NS124974), NCATS (R21TR003184), CDC (U01DD001242), and the FDA (7R01FD006071). He has received grant funding from the Myotonic Dystrophy Foundation, C3 Foundation, and the Muscular Dystrophy Association. He receives royalties from the CCMDHI and the CMTHI. He receives research funds from Takeda, Sanofi Genzyme, Dyne, Vertex Pharmaceuticals, Fulcrum Therapeutics, AskBio, ML Bio, Pfizer, and Sarepta. He has provided consultation for Arthex, Takeda, Dyne, Avidity, Regenta, and Vertex Pharmaceuticals. The other authors have no conflicts of interest.
Figures
References
-
- Guy‐Coichard C., Nguyen D. T., Delorme T., and Boureau F., “Pain in Hereditary Neuromuscular Disorders and Myasthenia Gravis: A National Survey of Frequency, Characteristics, and Impact,” Journal of Pain and Symptom Management 35, no. 1 (2008): 40–50, 10.1016/j.jpainsymman.2007.02.041. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- R21 TR003184/TR/NCATS NIH HHS/United States
- U54 NS053672/NS/NINDS NIH HHS/United States
- U01 DD001116/DD/NCBDD CDC HHS/United States
- U01 DD001242/DD/NCBDD CDC HHS/United States
- U01 DD001120/DD/NCBDD CDC HHS/United States
- R01 FD006071/FD/FDA HHS/United States
- U01 NS124974/NS/NINDS NIH HHS/United States
- U01 DD001255/DD/NCBDD CDC HHS/United States
- U01 DD001252/DD/NCBDD CDC HHS/United States
- CC/CDC HHS/United States
- U01 DD001117/DD/NCBDD CDC HHS/United States
- U01 DD001126/DD/NCBDD CDC HHS/United States
- U01 DD001123/DD/NCBDD CDC HHS/United States
- R01 NS104010/NS/NINDS NIH HHS/United States
- U01 DD001119/DD/NCBDD CDC HHS/United States
- U01 DD001108/DD/NCBDD CDC HHS/United States
- U01 DD001248/DD/NCBDD CDC HHS/United States
- U01 DD001054/DD/NCBDD CDC HHS/United States
- CC/CDC HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

