Pregnancy after orthotopic liver transplantation: a comprehensive review
- PMID: 40546267
- PMCID: PMC12179207
- DOI: 10.3389/frtra.2025.1581273
Pregnancy after orthotopic liver transplantation: a comprehensive review
Abstract
Background: Medical innovations and advancements, such as orthotopic liver transplantation (OLT) allow thousands of patients worldwide to live comfortably, despite previously life-threatening conditions. Procreation, one of the most powerful human instincts, drives the force behind the increasing popularity of pregnancies after OLT, with their numbers rising since the first documented case in 1976. Pregnancy post OLT remains a high-risk event, requiring careful management by a multidisciplinary team of hepatologists, obstetricians, transplant surgeons, and neonatologists. This review aims to synthesize current evidence on family planning, pregnancy management, and maternal and neonatal outcomes in women who have undergone OLT, based on studies indexed in PubMed up to December 2024.
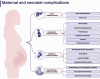
Findings: Due to ethical constraints, international registries of pregnancies after OLTs play a critical role in collecting observational data and establishing comprehensive guidelines for clinical practice. As the data indicated, OLT can help restore hormonal balance and menstrual cycle, enabling many women to conceive after OLT. However, adequate family planning is crucial, as women must be aware of the potential risks. Preconception counseling is essential to choose the right timing for pregnancy, assess graft function, and optimize immunosuppressive therapy, as some medications must be discontinued due to teratogenic risks. The risks associated with pregnancy in OLT recipients include gestational hypertension, preeclampsia, and gestational diabetes. Neonates are significantly more likely to experience prematurity and low birth weight. Post-partum management focuses on monitoring graft function, managing complications, and guiding breastfeeding.
Conclusions: Available literature and observational studies consistently demonstrate that women post-OLT can achieve successful pregnancies and deliver healthy infants. However, due to the inherent risks described in this population, such patients require specialized care from a multidisciplinary team. Further research is essential to optimize birth control methods and clarify the mechanisms behind the higher prevalence of pregnancy complications. Establishing the long-term safety data for immunosuppressive therapies, particularly regarding breastfeeding, is also needed.
Keywords: family planning in liver transplant recipients; immunosuppression during pregnancy; liver transplant and maternal outcomes; liver transplant and neonatal outcomes; post-transplant pregnancy risks; pregnancy after liver transplantation.
© 2025 Stelmach, Dery, Jabiry-Zieniewicz and Kupiec-Weglinski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Organ Procurement and Transplantation Network (OPTN). National data [Internet]. United Network for Organ Sharing (2024). Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ (Accessed January 14, 2025).
-
- Coscia L, Daly T, Nathan H, Armenti D, Kliniewski D, Constantinescu S, et al. Transplant pregnancy registry international. Transplantation. (2017) 101:S64. 10.1097/01.tp.0000525078.35784.d3 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

