The California Resuscitation Outcomes Consortium (CAL-ROC): A novel collaboration to facilitate the implementation of randomized clinical trials in the prehospital setting
- PMID: 40546306
- PMCID: PMC12179741
- DOI: 10.1016/j.resplu.2025.100992
The California Resuscitation Outcomes Consortium (CAL-ROC): A novel collaboration to facilitate the implementation of randomized clinical trials in the prehospital setting
Abstract
Background: Few large randomized clinical trials (RCTs) have been conducted to inform the prehospital phase of care for out-of-hospital cardiac arrest (OHCA). We describe the development of a consortium to facilitate large-scale prehospital RCTs through a novel research collaboration, leveraging pre-existing prehospital and hospital data capture infrastructure.
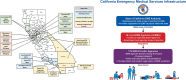
Consortium description: We developed a consortium consisting of 173 emergency medical services (EMS) Provider Agencies and four academic "hubs." The consortium is an innovative collaboration consisting of a diverse set of EMS experts from across California and designed to overcome logistical, cost, and regulatory challenges associated with prehospital research. All participating agencies share data via a state EMS database, California EMS Information System (CEMSIS), and contribute data to the national Cardiac Arrest Registry to Enhance Survival (CARES) database. Data from CEMSIS and CARES will be linked to capture RCT outcomes. We abstracted two years of data from the CARES database to characterize the population served by the consortium and facilitate sample size calculations for future trials. We estimate that the consortium will have the ability to enroll a diverse population of patients with OHCA, at a rate of approximately 19,000 per year across all sites, for a future trial of cardiac arrest therapies.
Conclusion: This collaboration uses pre-existing data infrastructure to capture prehospital and hospital outcome data to facilitate large-scale prehospital RCTs for time-critical emergencies.
Keywords: Cardiac arrest; Clinical trials; Emergency medical services; Out-of-hospital cardiac arrest.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Continuous chest compression versus interrupted chest compression for cardiopulmonary resuscitation of non-asphyxial out-of-hospital cardiac arrest.Cochrane Database Syst Rev. 2017 Mar 27;3(3):CD010134. doi: 10.1002/14651858.CD010134.pub2. Cochrane Database Syst Rev. 2017. PMID: 28349529 Free PMC article.
-
Prehospital Trauma Compendium: Prehospital Management of Adults with Traumatic Out-of-Hospital Circulatory Arrest - A Joint Position Statement and Resource Document of NAEMSP, ACS-COT, and ACEP.Prehosp Emerg Care. 2025 Mar 11:1-15. doi: 10.1080/10903127.2024.2428668. Online ahead of print. Prehosp Emerg Care. 2025. PMID: 40068140
-
Implementing an Educational Model for Cardiac Arrest Patients During Interfacility Transfers.Cureus. 2025 Mar 1;17(3):e79892. doi: 10.7759/cureus.79892. eCollection 2025 Mar. Cureus. 2025. PMID: 40171347 Free PMC article.
-
Surgery for epilepsy.Cochrane Database Syst Rev. 2015 Jul 1;(7):CD010541. doi: 10.1002/14651858.CD010541.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jun 25;6:CD010541. doi: 10.1002/14651858.CD010541.pub3. PMID: 26130264 Updated.
-
Extracorporeal membrane oxygenation for critically ill adults.Cochrane Database Syst Rev. 2015 Jan 22;1(1):CD010381. doi: 10.1002/14651858.CD010381.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2023 Sep 26;9:CD010381. doi: 10.1002/14651858.CD010381.pub3. PMID: 25608845 Free PMC article. Updated.
References
Grants and funding
LinkOut - more resources
Full Text Sources

