Oncolytic adeno-immunotherapy improves allogeneic adoptive HER2.CAR-NK function against pancreatic ductal adenocarcinoma
- PMID: 40546314
- PMCID: PMC12179663
- DOI: 10.1016/j.omton.2025.201006
Oncolytic adeno-immunotherapy improves allogeneic adoptive HER2.CAR-NK function against pancreatic ductal adenocarcinoma
Abstract
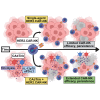
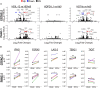
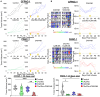
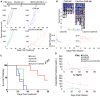
Pancreatic ductal adenocarcinoma (PDAC) responds poorly to conventional treatments and immunotherapy. We previously developed a binary oncolytic/helper-dependent adenovirus system (CAdTrio) that facilitated oncolysis and expressed the immunomodulatory molecule interleukin-12 and a programmed death ligand 1 (PD-L1) blocking mini-antibody. Given that CAdTrio enhanced endogenous natural killer (NK) cell anti-tumor activity in humanized mice bearing PDAC tumors and that NK cells can be adoptively transferred to patients safely in the allogeneic setting, we hypothesized that a combination of CAdTrio and allogeneic NK cells expressing a HER2-specific chimeric antigen receptor (HER2.CAR-NK) would be an effective, entirely "off-the-shelf" treatment against PDAC. We found that CAdTrio-derived immunomodulatory molecules prolonged HER2.CAR-NK persistence at tumor sites, allowing long-term tumor growth control and improved survival in both humanized mice and a heterogeneous PDAC patient-derived xenografts (PDX) model. This effect was based on CAdTrio-derived transgene support that shifted HER2.CAR-NK gene expression to that resembling an NK memory-like phenotype. Additionally, this allogeneic combination therapy was tolerated in humanized mice. Together, these data suggest that CAdTrio and HER2.CAR-NK cell combination immunotherapy may be a novel and effective option for the treatment for immunologically "cold" PDAC tumors.
Keywords: CAR-NK cell; MT: Regular Issue; PDX model; humanized mouse model; oncolytic viro-immuno therapy; pancreatic ductal adenocarcinoma.
© 2025 The Author(s).
Conflict of interest statement
M.S. received research funding from Tessa Therapeutic Ltd. and AstraZeneca. M.S. was a scientific consultant and C.P was a consultant for Tessa Therapeutic Ltd.
Figures


References
-
- Orth M., Metzger P., Gerum S., Mayerle J., Schneider G., Belka C., Schnurr M., Lauber K. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019;14:141. doi: 10.1186/s13014-019-1345-6. - DOI - PMC - PubMed
-
- Harder J., Ihorst G., Heinemann V., Hofheinz R., Moehler M., Buechler P., Kloeppel G., Röcken C., Bitzer M., Boeck S., et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br. J. Cancer. 2012;106:1033–1038. doi: 10.1038/bjc.2012.18. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
