A retrospective study to assess the accuracy of syphilis rapid test by chemiluminescent microparticle immunoassay (CIA) in volunteer blood donors
- PMID: 40546366
- PMCID: PMC12180880
- DOI: 10.4103/ijstd.ijstd_115_24
A retrospective study to assess the accuracy of syphilis rapid test by chemiluminescent microparticle immunoassay (CIA) in volunteer blood donors
Abstract
Background: Syphilis screening methods in blood banks have recently transitioned from manual nontreponemal tests to automated treponemal-specific enzyme immunoassays and chemiluminescence immunoassays (CIA). Although convenient and highly sensitive, this has led to increased false-positive rates leading to the wastage of blood resources.
Aims and objectives: This study aimed to evaluate the positive predictive value (PPV) of Treponema pallidum (TP)-CIA as a screening tool in healthy blood donors and determine optimal cutoff values to improve specificity, thereby reducing unnecessary blood wastage and donor exclusion.
Patients and methods: It was a retrospective observational study that collected data from July 2019 to May 2023 on blood bank donors referred to the sexually transmitted disease clinic, in view of testing reactive on the Abbott ARCHITECT Syphilis TP CMIA (relative light unit [RLU] >1). Clinical details, Venereal Disease Research Laboratory (VDRL) titers, and confirmatory TP hemagglutination (TPHA) test records were corroborated.
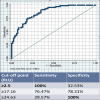
Results: A total of 1791 patients were referred over 4 years as per blood bank records. Only 271 (15.13%) of these presented to the sexually transmitted infection clinic, indicating a high referral loss. Of patients with CIA titers available, 119/202 (58.9%) were diagnosed as confirmed syphilis based on VDRL and TPHA positivity, out of which majority (92.4%) were latent syphilis. PPV of TP-CIA rapid test at RLU >1 (manufacturer recommendation) was 64.3%, with an area under the receiver operating characteristic curve of 0.86. Setting the cutoff at RLU ≥2.5 improved sensitivity to 100% (patients with RLU <2.5 could be safely returned to the donor pool), while a cutoff of ≥24.63 achieved 100% specificity (confirmed syphilis).
Conclusion: The manufacturer cutoff for syphilis CMIA rapid test (RLU >1) maintains high sensitivity but generates high false positives, causing nearly one-third of patients to be referred unnecessarily, as well as blood wastage. Reflexive confirmatory testing with a second treponemal assay can minimize the psychological impact on healthy donors and prevent unnecessary donor exclusion. Large-scale studies are required to establish population-based cutoffs for ensured blood safety without wastage.
Keywords: Blood bank; chemiluminescent immunoassay; reverse screening algorithm; syphilis; treponemal tests.
Copyright: © 2025 Indian Journal of Sexually Transmitted Diseases and AIDS.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Diagnostics Direct. LLC. 510(k) Substantial Equivalence Determination Decision Summary: Syphilis Health Check Treponemal Antibody Test. 2010 510(k) Number: K102400. FDA [monograph on the Internet]
-
- Centers for Disease Control and Prevention (CDC). Discordant results from reverse sequence syphilis screening –Five laboratories, United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011;60:133–7. - PubMed
-
- Mehta N, Bhari N, Gupta S. Asian guidelines for syphilis. J Infect Chemother. 2022;28:1084–91. - PubMed
-
- Food and Drug Administration. Recommendations for Screening, Testing and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis. Silver Spring, MD: FDA-CBER; 2020.
LinkOut - more resources
Full Text Sources