Drug-Induced Acute Liver Injury Due to Tamoxifen Prophylaxis
- PMID: 40546519
- PMCID: PMC12180419
- DOI: 10.7759/cureus.84552
Drug-Induced Acute Liver Injury Due to Tamoxifen Prophylaxis
Abstract
A 41-year-old female was diagnosed with stage II ductal breast cancer in November 2023. The patient subsequently underwent a bilateral mastectomy and was started on tamoxifen 20 mg as post-operative adjuvant therapy in December 2023. Surveillance positron emission tomography (six months post-mastectomy) confirmed no cancer recurrence prior to symptom onset. Following abdominal pain, nausea and vomiting, pruritus, and jaundice, in the context of an elevated bilirubin level and a 1.5 mm gallstone, tamoxifen treatment was discontinued in July 2024. In August 2024, the patient presented with recurrent nausea and vomiting, increased abdominal pain, worsening pruritus and jaundice, debilitating back pain, and a weight loss of 20.87 kg. The patient was admitted to the hospital for further workup. Her bilirubin levels were found to be 17.9 mg/dl (normal range: 0.1-1.2 mg/dL). Following subsequent labs and scans such as a hepatobiliary iminodiacetic acid scan, liver biopsy, computerized tomography of the lumbar spine, magnetic resonance imaging of the lumbar spine and bilateral hips, anti-smooth muscle antibody test, and more diagnostic testing, it was determined that the patient was suffering from drug-induced liver injury. After the initiation of steroids and ursodiol, her bilirubin measurements decreased. The patient reported resolution of symptoms a month later, as her bilirubin levels had declined from 17.9 to 2.3 mg/dL over four weeks. This unique presentation of tamoxifen-induced acute liver injury is reversible, and patients on tamoxifen need a frequent comprehensive metabolic panel to detect possible liver injury preemptively.
Keywords: bilirubin; cholestatic jaundice; drug-induced liver injury (dili); her-2 positive breast cancer; tamoxifen therapy.
Copyright © 2025, Nichani et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
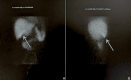
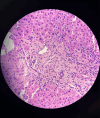
References
-
- Tamoxifen-induced hepatotoxicity via lipid accumulation and inflammation in zebrafish. Yu Q, Huo J, Zhang Y, et al. Chemosphere. 2020;239:124705. - PubMed
-
- Francis P, Navarro VJ. StatPearls. Treasure Island (FL): StatPearls; 2024. Drug-Induced Hepatotoxicity. - PubMed
-
- Feldman M, Friedman LS, Brandt LJ. Elsevier; 2015. Sleisenger and Fordtran's Gastrointestinal and Liver Disease.
-
- ACG clinical guideline: diagnosis and management of idiosyncratic drug-induced liver injury. Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR. Am J Gastroenterol. 2021;116:878–898. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
