Diarylpentanoid, a curcumin analog, inhibits malignant meningioma growth in both in vitro and in vivo models
- PMID: 40546670
- PMCID: PMC12019622
- DOI: 10.5493/wjem.v15.i2.102897
Diarylpentanoid, a curcumin analog, inhibits malignant meningioma growth in both in vitro and in vivo models
Abstract
Background: Malignant meningioma metastasizes systemically, primarily due to its role in epithelial-mesenchymal transition. Although the prognosis is extremely poor, drug development efforts have been limited, because this tumor is categorized as a rare form.
Aim: To examine growth suppressive effect of GO-Y030, a diarylpentanoid curcumin analog, (1E,4E)-1,5-bis [3,5-bis (methoxymethoxy) phenyl] penta-1,4-dien-3-one against the malignant meningioma.
Methods: The growth suppression of malignant meningioma cells by GO-Y022 and GO-Y030 were examined, using IOMM-Lee and HKBMM cell lines. Male nude mice aged eight weeks, specifically BALB/cSlc-nu/nu mice received a subcutaneous inoculation of IOMM-Lee (107 cells/site) on their back and 30 μg/kg of recombinant hepatocellular growth factor (HGF) was injected into the tumor every three days. After confirmed the growth tumor mass, 500 μL of GO-Y030 diluted with PBS were administrated intraperitoneally daily at doses of 1 mg/kg and 2 mg/kg, respectively.
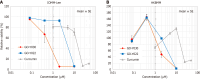
Results: GO-Y030 exhibits a growth inhibitory effect on malignant meningioma cell lines, IOMM-Lee and HKBMM ranging from 0.8-2.0 μM in vitro. Notably, GO-Y030's inhibitory effect is about 10 to 16th times more potent than that of curcumin, which has previously demonstrated potential in combating malignant meningioma. In mouse models, the intraperitoneal administration of GO-Y030 effectively suppresses the growth of malignant meningioma tumors that have been inoculated in the back (P = 0.002). High-performance liquid chromatography analysis has confirmed the distribution of GO-Y030 in the bloodstream and brain tissue. Moreover, GO-Y030 demonstrates the ability to significantly suppress HGF (P < 0.01), nuclear factor kappa B (P < 0.001), and N-cadherin (P < 0.001), all of which contribute to the epithelial-mesenchymal transition.
Conclusion: GO-Y030 holds promise as a potent compound for the systemic inhibition of malignant meningioma. GO-Y030 has higher tumor growth inhibitory effect against meningiomas than curcumin, which is known to have antitumor activity through multi-molecular target control resulting in apoptosis induction. GO-Y030 controls at least three molecules of HGF, nuclear factor kappa B, and N-cadherin.
Keywords: Curcumin; Diarylpentanoid curcumin analog; Hepatocellular growth factor; Malignant meningioma; N-cadherin; Nuclear factor kappa B.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures





Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Fountain DM, Young AMH, Santarius T. Malignant meningiomas. Handb Clin Neurol. 2020;170:245–250. - PubMed
-
- Schrell UM, Rittig MG, Anders M, Koch UH, Marschalek R, Kiesewetter F, Fahlbusch R. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997;86:840–844. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

