Diagnostic testing for chronic spontaneous urticaria with or without angioedema: The do's, don't and maybe's
- PMID: 40546743
- PMCID: PMC12180992
- DOI: 10.1016/j.waojou.2025.101068
Diagnostic testing for chronic spontaneous urticaria with or without angioedema: The do's, don't and maybe's
Abstract
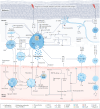
Chronic spontaneous urticaria (CSU), with or without angioedema, is heterogeneous and comprised of different endotypes and phenotypes. Because acute urticaria will mostly resolve spontaneously, routine testing and laboratory evaluation is not required unless supported by the clinical history or physical examination. With the advent of omalizumab, there has been a surge of interest in identifying biomarkers that could predict response to this treatment. In the process of investigating biomarkers as prognosticators, several CSU phenotypes and endotypes have emerged, which have made it evident that novel therapies targeting non-IgE mechanistic pathways are needed to control symptoms in patients unresponsive to the currently recommended therapies by the most recent international guidelines. The current data support peripheral eosinophils, autoantibodies against IgE or FcεRI α subunit measured by basophil histamine release assays, total IgE levels and IgG autoantibodies against thyroid peroxidase (TPO) as specific markers to differentiate type 1 autoimmune (autoallergic) CSU from type 2b autoimmune CSU before starting treatment especially with omalizumab. These markers have been included as exploratory endpoints in many clinical trials investigating novel therapies or for repurposing existing biologics to determine responders and non-responders, but these data are not completely clear at this time. Therefore, further randomized controlled studies and real-world studies are needed to demonstrate more conclusively the utility of ordering these tests in CSU patients when they initially present or when it is determined they are not responsive to high dose second generation H1-antihistamines (SGAH) before they can be included in evidence-based CSU guidelines. This review examines the value of obtaining diagnostic tests in the initial evaluation of CSU patients to predict treatment response.
Keywords: Angioedema; Biomarkers; Chronic spontaneous urticaria; Endotypes; Phenotypes; Treatment responders.
© 2025 The Authors.
Conflict of interest statement
JB: PI/consultant: Novartis, Genentech, Sanofi-Regeneron, Allakos, Celldex, Jasper, AZ, Amgen, Escient/Incyte, Evoimmune, Takeda/Shire, CSL Behring, Biocyrst, Kalvista, Pharvaris, Ionis, Intellia, Biomarin, Astria, Blueprint Medicine, Cogent, Telios. IJA reports personal fees from Bayer, Bial, Cipla, Eurodrug, Gebro, Glenmark, Menarini, MSD, Roxall and Sanofi outside the submitted work. RA has been speaker and/or consultant for Novartis, Sanofi, Jasper Therapeutcs, GSK, HAL Allergy, Allergy Therapeutics, Smart Practice. AB: Research Grant: Intellia, Ionis, Astria; Advisory Board: CSL, Ionis, Pharvaris, Intellia, Kalvista, Astria, Takeda, ADARx. SB: advisory Board or equivalent for Astria, Biocryst, CSL Behring, Ionis, Kalvista, Pharvaris, Sanofi, Takeda; Grants or honoraria: Biocryst, CSL Behring, Kalvista, Takeda; Participating or participated in a clinical trial: Takeda. TC: Research-CSL Behring, Takeda, Astria, Kalvista, Pharvaris, Ionis, GSK, Sanofi; Speaking- Sanofi, Regeneron; Honorarium- CSL Behring. Takesa, Grifols, Kalvista. RMG: Former speaker Novartis. AB: Research Grant: Intellia, Ionis, Astria; Advisory Board: CSL, Ionis, Pharvaris, Intellia, Kalvista, Astria, Takeda, ADARx. HJL: has participated in research with, served as a speaker or advisor for, participated in educational projects with or received educational grants from the following: Astria, CSL Behring, Intellia, KalVista, Pharvaris, Takeda. MH: has been a speaker and/or advisor for and has received honoraria from Kaken, Kyorin, Kyowa Kirin, Mitsubishi-Tanabe, Novartis, Sanofi/Regeneron, Taiho, and Teikoku. ASG: receives a productivity scholarship from Brazilian National Council of Research (CNPq); she received research funding, honoraria for educational activities or acted as a consultant for Catalyst Pharmaceuticals, CSL Behring, Takeda, Kalvista, Pharvaris, Pint-Pharma, Multicare, Biomarin, Binding-site, Kedrion and Astra. DL: David Lang has carried out clinical research with, served as a consultant for, and/or has received honoraria from: AstraZeneca, Blueprint, Celldex, Genentech, Novartis, Sanofi-Regeneron. HP: reports fees for lectures or advisory board participation (last three years) from AstraZeneca, FAES Farma, GSK, JABA Recordati, Leti, Medinfar, Menarini, Organon, Stallergenes, Tecnimede, Viatris; clinical trials paid to her Institution from Novartis and AstraZeneca. MR: has carried out clinical research with, served as a consultant for, and/or has received honoraria from: Astria, Biocryst, Biomarin, Celldex, CSL Behring, Cycle Pharma, Grifols, Intellia, Ionis, Kalvista, Novartis, Pharming, Pharvaris, Sanofi-Regeneron, Takeda Celldex, Novartis, Sanofi-Regeneron. SSS: NIH, Novartis, Escient, Allakos, Jasper, Celldex, Allakos, Granular Therapeutics, Genentech, Celldex, Evommune, Novartis, Escient, Celltrion, Sanofi, Nucor, GSK. GS: has received research support from Aimmune, Amgen, Astra-Zeneca, DBV technologies, Genentech, Leo Pharma Novartis,Sanofi, Regeneron and ALK; is a medical advisor for Novartis, CSL Behring, Pfizer, Abvie, Astra-Zeneca, Nuvo Pharmaceuticals, Eli Lilly, Incyte and the Allergy Asthma and Immunology Society of Ontario. AZ: has received honoraria, meeting/travel support, and/or served on advisory boards for Astria, BioCryst, CSL Behring, Intellia, KalVista Pharmaceuticals, Otsuka, Pharvaris, and Takeda. TZ: reports honoraria for lectures from Amgen, AstraZeneca, AbbVie, ALK -Abelló, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, FAES Farma, HAL Allergie GmbH, Henkel, Kryolan, Leti, L'Oreal, Meda, Menarini, Merck Sharp & Dohme, Novartis, Nuocor, Pfizer, Sanofi, Stallergenes, Takeda, Teva, UCB, and Uriach; Fees for industry consulting were received from Abivax, Almirall,Blueprint, Celldex, Celltrion, Novartis, and Sanofi. BZ: Consultant - CSL Behring, Takeda/Shire, Biocyrst, Novartis, Genentech, Biomarin; Laboratory agreement – Ionis. ML, JLGA, MMA, DVN, MIRJ, ET: nothing to report.
Figures


References
-
- Zuberbier T., Abdul Latiff A.H., Abuzakouk M., et al. The international EAACI/GA(2)LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77(3):734–766. - PubMed
-
- Metz M., Altrichter S., Buttgereit T., et al. The diagnostic workup in chronic spontaneous urticaria-what to test and why. J Allergy Clin Immunol Pract. 2021;9(6):2274–2283. - PubMed
-
- Bernstein J.A., Lang D.M., Khan D.A., et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270–1277. - PubMed

