Extracellular Vesicle-Integrated Biomaterials in Bone Tissue Engineering Applications: Current Progress and Future Perspectives
- PMID: 40546799
- PMCID: PMC12182063
- DOI: 10.2147/IJN.S522198
Extracellular Vesicle-Integrated Biomaterials in Bone Tissue Engineering Applications: Current Progress and Future Perspectives
Abstract
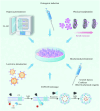
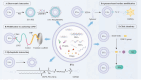
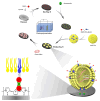
With an aging population and increased life expectancy, the clinical burden of bone-related disorders, especially large bone defects, continues to grow, underscoring the urgent need for effective regenerative strategies. Effective bone regeneration is essential not only for restoring skeletal structure and function but also for improving patients' quality of life and reducing the socioeconomic burden associated with prolonged recovery or surgical failure. Bone tissue engineering has emerged as a promising approach for healing large bone defects. Traditionally, stem cells, biomaterial scaffolds and growth factors have been considered the three essential elements of bone tissue engineering. However, stem cell-based therapies face several significant challenges, including ectopic tissue formation, malignant transformation, cell embolism, and immune rejection. In recent years, extracellular vesicles (EVs) have gained significant attention as an advanced alternative to stem cells and a novel cell-free therapy for bone regeneration due to their inherent advantages, such as low immune-rejection, excellent biocompatibility, significant bioactivity and high feasibility for carrying bioactive molecules or drugs. This review provides a comprehensive overview of the current state and future potential of EV-based strategies in bone tissue engineering. We first review the sources of parent cells for EVs applied in bone tissue engineering and the roles and potential mechanisms of EVs in bone regeneration. We then discuss the various modification strategies employed to enhance the therapeutic potential of EVs. Additionally, we summarize strategies for integrating EVs with various biomaterial scaffolds, with a specific focus on the latest advances in achieving controlled and sustained release of EVs from scaffolds at bone defect sites. Collectively, this review aims to offer key insights into the translational potential of EV-functionalized biomaterials and guide future directions in the development of next-generation bone regenerative therapies.
Keywords: biomaterials; bone tissue engineering; extracellular vesicles; mesenchymal stem cells.
© 2025 Huang and Xie.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Extracellular vesicles derived from Schwann cells to enhance bone and dental tissue regeneration: a literature review.J Nanobiotechnology. 2025 Jul 11;23(1):502. doi: 10.1186/s12951-025-03585-7. J Nanobiotechnology. 2025. PMID: 40646600 Free PMC article. Review.
-
Mineralized osteoblast-derived exosomes and 3D-printed ceramic-based scaffolds for enhanced bone healing: A preclinical exploration.Acta Biomater. 2025 Jun 15;200:686-702. doi: 10.1016/j.actbio.2025.05.051. Epub 2025 May 21. Acta Biomater. 2025. PMID: 40409510
-
Property-tailoring chemical modifications of hyaluronic acid for regenerative medicine applications.Acta Biomater. 2025 Jul 1;201:75-100. doi: 10.1016/j.actbio.2025.06.014. Epub 2025 Jun 7. Acta Biomater. 2025. PMID: 40490241 Review.
-
Computational Fluid Dynamics Modeling of Material Transport Through Triply Periodic Minimal Surface Scaffolds for Bone Tissue Engineering.J Biomech Eng. 2025 Mar 1;147(3):031007. doi: 10.1115/1.4067575. J Biomech Eng. 2025. PMID: 39790065
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

