Advancements, Challenges, and Future Prospects of Nanotechnology in Sepsis Therapy
- PMID: 40546802
- PMCID: PMC12180596
- DOI: 10.2147/IJN.S488026
Advancements, Challenges, and Future Prospects of Nanotechnology in Sepsis Therapy
Abstract
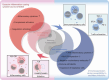
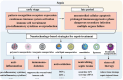
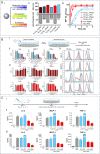
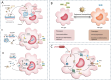
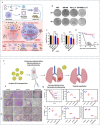
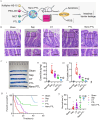
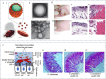
Sepsis is a life-threatening systemic inflammatory syndrome, typically triggered by infection, that can lead to multi-organ failure and high mortality rates. Traditional treatments for sepsis often have limited efficacy and significant side effects, necessitating the exploration of innovative therapeutic strategies. In recent years, the application of nanotechnology in sepsis therapy has garnered widespread attention due to its potential to modulate immune responses, reduce inflammation and oxidative stress, and eliminate bacterial toxins. This review aims to provide an overview of the latest advancements, challenges, and future prospects of nanotechnology in sepsis treatment. By analyzing recent developments in anti-inflammatory, immunomodulatory, antioxidant, and detoxification applications of nanotechnology, key findings and therapeutic potential are summarized, including the use of nanocarriers, biomimetic nanoparticles, and self-assembled nanomaterials. Furthermore, this review addresses the challenges in clinical translation, such as drug targeting, long-term safety, and biocompatibility. Future research will require large-scale clinical trials and interdisciplinary collaboration to validate the efficacy of nanotechnology in sepsis treatment and facilitate its integration into clinical practice. Overall, nanotechnology presents unprecedented opportunities for sepsis management, and this review seeks to offer insights into ongoing research while promoting further advancements in this field.
Keywords: MODS; nanoimmunotherapy; nanomedicine delivery methods; nanotechnology; sepsis.
© 2025 Liu et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

