Acute kidney injury through a metabolic lens: pathological reprogramming mechanisms and clinical translation potential
- PMID: 40546823
- PMCID: PMC12178853
- DOI: 10.3389/fphys.2025.1602865
Acute kidney injury through a metabolic lens: pathological reprogramming mechanisms and clinical translation potential
Abstract
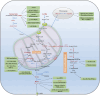
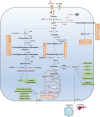
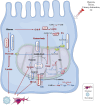
Acute kidney injury (AKI) represents a clinical syndrome with a bleak short-term prognosis, posing a high risk for the development of chronic kidney diseases and end-stage kidney disease. The underlying mechanisms of AKI are still not fully understood, and effective intervention strategies remain elusive. Enormous energy is required to meet the functional activity in hypermetabolic tubular epithelial cells (TECs), the most vulnerable cell types during AKI. Recent evidence has shed light on the reprogramming of metabolic pathways and the shift in energy substrates under pathological conditions. The reprogrammed metabolic pathway initially serves to compensate for energy shortages and supply substrates for cell repair during the early stages of AKI. However, sustained metabolic dysregulation tend to become detrimental for tubular repair and regeneration. Intriguingly, dynamic alterations in specific metabolites extend beyond their conventional roles as metabolic byproducts, actively participating in pathophysiology through multifaceted regulatory mechanisms during AKI. As yet, clinical therapy for AKI has not yet incorporated the intervention of metabolic disorders, highlighting a vast potential for extensive application. This review aims to summarize recent studies on the role of metabolic pathway reprogramming and metabolites in AKI, while discussing promising therapeutic strategies targeting metabolic reprogramming.
Keywords: BCAAs; acute kidney injury; fatty acid oxidation; glutaminolysis; glycolysis; ketolysis.
Copyright © 2025 Gao, Huang, Zhang, Wei, Yu, Xing, Yuan, Ning and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Timing of kidney replacement therapy initiation for acute kidney injury.Cochrane Database Syst Rev. 2022 Nov 23;11(11):CD010612. doi: 10.1002/14651858.CD010612.pub3. Cochrane Database Syst Rev. 2022. PMID: 36416787 Free PMC article.
-
Non-pharmacological interventions for preventing clotting of extracorporeal circuits during continuous renal replacement therapy.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD013330. doi: 10.1002/14651858.CD013330.pub2. Cochrane Database Syst Rev. 2021. PMID: 34519356 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
The use of Open Dialogue in Trauma Informed Care services for mental health consumers and their family networks: A scoping review.J Psychiatr Ment Health Nurs. 2024 Aug;31(4):681-698. doi: 10.1111/jpm.13023. Epub 2024 Jan 17. J Psychiatr Ment Health Nurs. 2024. PMID: 38230967
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

