PTEN protein phosphatase activity regulates metastasis by targeting PKCδ
- PMID: 40546936
- PMCID: PMC12179624
- DOI: 10.1016/j.isci.2025.112741
PTEN protein phosphatase activity regulates metastasis by targeting PKCδ
Abstract
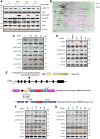
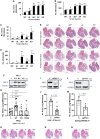
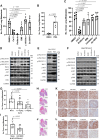
PTEN acts as a tumor suppressor through its lipid and protein phosphatase activities. We previously reported that PTEN phosphatase inhibits metastasis independent of its lipid phosphatase. To determine PTEN phosphatase downstream substrates and their role precisely in metastatic suppression, we used proteomic approaches to identify PKCδ as PTEN protein phosphatase substrates. We show that the inactivation of PTEN protein phosphatase activity causes loss of the capability to dephosphorylate PKCδ at S643, T505, and Y311, but wild-type or PTEN lipid phosphatase deficient mutants can maintain. We then established knock-in and knock-out models to confirm that PTEN protein phosphatase is required to inhibit PKCδ phosphorylation and necessary to suppress tumor metastasis. Notably, we found that PKCδ could promote metastasis of melanoma cells with wild-type PTEN. Still, the knockdown of PKCδ abrogated the metastatic potential of PTEN phosphatase-deficient melanoma cells, linking PTEN metastasis suppressor function to PTEN protein phosphatase and its substrate PKCδ.
Keywords: Proteomics; cancer.
© 2025 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Interventions to prevent occupational noise-induced hearing loss.Cochrane Database Syst Rev. 2017 Jul 7;7(7):CD006396. doi: 10.1002/14651858.CD006396.pub4. Cochrane Database Syst Rev. 2017. PMID: 28685503 Free PMC article.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
-
Hepatoma-Derived Growth Factor Promotes Liver Carcinogenesis by Inducing Phosphatase and Tensin Homolog Inactivation.Lab Invest. 2025 Jun;105(6):104127. doi: 10.1016/j.labinv.2025.104127. Epub 2025 Mar 11. Lab Invest. 2025. PMID: 40081662
References
LinkOut - more resources
Full Text Sources
Research Materials

