Mucosal boosting increases protective efficacy of an influenza vaccine in mice
- PMID: 40546962
- PMCID: PMC12179630
- DOI: 10.1016/j.isci.2025.112721
Mucosal boosting increases protective efficacy of an influenza vaccine in mice
Abstract
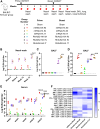
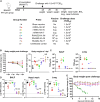
Influenza virus infection is a significant cause of global mortality. However, the development of influenza vaccines that induce robust immune responses at the site of respiratory mucosal exposure has proven challenging. Here, we assessed immune responses and protective efficacy of a rhesus adenovirus serotype 52 (RhAd52) vectored influenza vaccine encoding the hemagglutinin (HA) glycoprotein from A/California/07/2009 administrated by systemic or mucosal routes of immunization. We observed robust and durable systemic and mucosal immunity, including IgA and tissue resident memory T cells in the respiratory mucosa, in mice that received the vaccine intranasally or intratracheally. In contrast, only systemic immune responses were observed in mice that received the vaccine intramuscularly. Moreover, a single intranasal or intratracheal dose of RhAd52-HA provided near complete protection against a lethal challenge with a mouse-adapted influenza virus strain, whereas intramuscular immunization with RhAd52-HA and mRNA-HA provided less robust protection. Our data demonstrate the importance of mucosal immunity for enhancing vaccine protection against influenza.
Keywords: Immunity; Virology.
© 2025 The Authors.
Conflict of interest statement
D.H.B. is a co-founder and holds equity in Vector Sciences.
Figures



Similar articles
-
Adenovirus and mRNA vaccines as well as mucosal boosting improve protective efficacy against influenza virus challenge in macaques.Sci Transl Med. 2025 Jul 9;17(806):eadu7646. doi: 10.1126/scitranslmed.adu7646. Epub 2025 Jul 9. Sci Transl Med. 2025. PMID: 40632835
-
Binding antibody titers against the hemagglutinin and neuraminidase correlate with protection against medically attended influenza A and B disease.J Virol. 2025 Jun 17;99(6):e0039125. doi: 10.1128/jvi.00391-25. Epub 2025 May 13. J Virol. 2025. PMID: 40358209 Free PMC article.
-
Research Advances for Virus-vectored Tuberculosis Vaccines and Latest Findings on Tuberculosis Vaccine Development.Front Immunol. 2022 Jun 23;13:895020. doi: 10.3389/fimmu.2022.895020. eCollection 2022. Front Immunol. 2022. PMID: 35812383 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Influenza vaccine for chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2018 Jun 26;6(6):CD002733. doi: 10.1002/14651858.CD002733.pub3. Cochrane Database Syst Rev. 2018. PMID: 29943802 Free PMC article.
References
-
- Weekly U.S Influenza Surveillance Report. 2024. https://www.cdc.gov/flu/weekly/index.htm
-
- Influenza (Seasonal) 2023. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- CDC Seasonal Flu Vaccine Effectiveness Studies. 2024. https://www.cdc.gov/flu-vaccines-work/php/effectiveness-studies/index.html
-
- Zeno E.E., Nogareda F., Regan A., Couto P., Rondy M., Jara J., Voto C., Rojas Mena M.P., Katz N., del Valle Juarez M., et al. Interim Effectiveness Estimates of 2024 Southern Hemisphere Influenza Vaccines in Preventing Influenza-Associated Hospitalization — REVELAC-i Network, Five South American Countries March–July 2024. MMWR Morb. Mortal. Wkly. Rep. 2024;73:861–868. doi: 10.15585/mmwr.mm7339a1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

