The complexity and commonness of the two-process model of sleep regulation from a mathematical perspective
- PMID: 40546978
- PMCID: PMC12176636
- DOI: 10.1038/s44323-025-00039-z
The complexity and commonness of the two-process model of sleep regulation from a mathematical perspective
Abstract
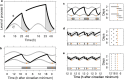
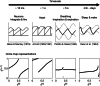
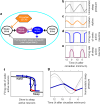
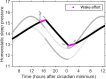
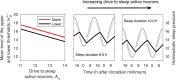
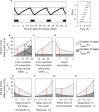
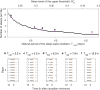
The two-process model (2pm) of sleep regulation is a conceptual framework and consists of mathematical equations. It shares similarities with models for cardiac, respiratory and neuronal rhythms and falls within the wider class of coupled oscillator models. The 2pm is related to neuronal mutual inhibition models of sleep-wake regulation. The mathematical structure of the 2pm, in which the sleep-wake cycle is entrained to the circadian pacemaker, explains sleep patterns in the absence of 24 h time cues, in different species and in early childhood. Extending the 2pm with a process describing the response of the circadian pacemaker to light creates a hierarchical entrainment system with feedback which permits quantitative modelling of the effect of self-selected light on sleep and circadian timing. The extended 2pm provides new interpretations of sleep phenotypes and provides quantitative predictions of effects of sleep and light interventions to support sleep and circadian alignment in individuals, including those with neurodegenerative disorders.
Keywords: Circadian mechanisms; Circadian regulation; Circadian rhythms and sleep; Sleep; Sleep deprivation; Slow-wave sleep.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsA.C.S. has no competing interests. D.J.D. is a consultant to Boehringer Ingelheim and Astronautx, and collaborates and/or has received equipment from SomnoMed and VitalThings.
Figures


Similar articles
-
Non-pharmacological interventions for sleep promotion in hospitalized children.Cochrane Database Syst Rev. 2022 Jun 15;6(6):CD012908. doi: 10.1002/14651858.CD012908.pub2. Cochrane Database Syst Rev. 2022. PMID: 35703367 Free PMC article.
-
Melatonin for the promotion of sleep in adults in the intensive care unit.Cochrane Database Syst Rev. 2018 May 10;5(5):CD012455. doi: 10.1002/14651858.CD012455.pub2. Cochrane Database Syst Rev. 2018. PMID: 29746721 Free PMC article.
-
Sleep waves and recovery from drug and alcohol dependence: Towards a rhythm analysis of sleep in residential treatment.Soc Sci Med. 2017 Jul;184:124-133. doi: 10.1016/j.socscimed.2017.05.016. Epub 2017 May 6. Soc Sci Med. 2017. PMID: 28525781 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
Non-invasive positive pressure ventilation for central sleep apnoea in adults.Cochrane Database Syst Rev. 2022 Oct 24;10(10):CD012889. doi: 10.1002/14651858.CD012889.pub2. Cochrane Database Syst Rev. 2022. PMID: 36278514 Free PMC article.
Cited by
-
Sleep pressure is differentially regulated by molecularly distinct subtypes of Lhx6-positive and Lhx6-negative neurons of the zona incerta.bioRxiv [Preprint]. 2025 Aug 1:2025.07.30.667705. doi: 10.1101/2025.07.30.667705. bioRxiv. 2025. PMID: 40766484 Free PMC article. Preprint.
References
-
- Borbély, A. A. A two process model of sleep regulation. Hum. Neurobiol.1, 195–204 (1982). - PubMed
-
- Daan, S., Beersma, D. G. M. & Borbély, A. A. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am. J. Physiol.246, R161–R183 (1984). - PubMed
-
- Borbély, A. A. & Wirz-Justice, A. Sleep, sleep deprivation and depression. a hypothesis derived from a model of sleep regulation. Hum. Neurobiol.1, 205–210 (1982). - PubMed
LinkOut - more resources
Full Text Sources
