Lymphocyte exhaustion in hepatocellular carcinoma: a dynamic evolution across disease stages
- PMID: 40547009
- PMCID: PMC12179174
- DOI: 10.3389/fimmu.2025.1611365
Lymphocyte exhaustion in hepatocellular carcinoma: a dynamic evolution across disease stages
Abstract
Background: Immune checkpoint inhibitors (ICIs) have transformed cancer therapy. However, their efficacy in hepatocellular carcinoma (HCC) is limited, highlighting the need to further explore immune microenvironments and novel biomarkers. This study examined lymphocyte populations and immune checkpoint dynamics in early, advanced, and post-progression HCC to better understand immune dynamics in HCC and to help identify predictive biomarkers and immune modulation strategies.
Methods: Tumoral and non-tumoral liver tissues were analyzed from HCC patients across early (n=25), advanced (n=22), and advanced-beyond-progression (n=15) stages. Lymphocyte profiling was performed using immunohistochemistry and flow cytometry, focusing on NK cells, T cells, and immune exhaustion markers. An exploratory analysis of this profile and its association with disease progression and recurrence was conducted.
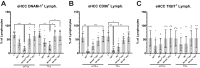
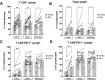
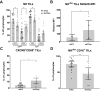
Results: Early HCC exhibited higher liver-resident NK (lrNK) cell densities in non-tumor regions, which diminished with advanced stages. Increased CD56+ cell infiltration in the tumor core was associated with recurrence. Tumor region showed elevated PD-1, NKG2A, and CD39 expression in CD4+ and CD8+ T cells, indicating progressive immune exhaustion. Advanced HCC stages demonstrated altered NK cell phenotypes, with reduced cytotoxic activation (CD16) and increased residency markers (CXCR6/CD69) in tumor-isolated lymphocytes.
Conclusions: Progressive immune exhaustion and dysregulation of lrNK and T cells in HCC reflect the evolution of the immune microenvironment originating in the tumor and leaking into the non-tumoral liver, progressively diminishing the cytotoxic capacity of NK and T cells. CD56+ cell density and immune checkpoint profiles are potential biomarkers for therapeutic response and disease monitoring, underscoring the need for personalized immunotherapy strategies.
Keywords: NK cells; hepatocellular carcinoma; immune exhaustion; lymphocytes; sorafenib.
Copyright © 2025 Fuster-Anglada, Corominas, Marsal, Llarch, Iserte, Sanduzzi-Zamparelli, Forner, Ferrer-Fábrega, Holguin, Morales, Saavedra, Reig, Boix, Marí and Díaz.
Conflict of interest statement
MR: Employment: Head of Liver Oncology Unit. Hospital Clinic Barcelona. Director of BCLC group at IDIBAPS/CIBEREHD. Consultant or Advisory Role: AstraZeneca, Bayer, BMS, Eli Lilly, Geneos, Ipsen, Merck, Roche, Universal DX, Boston Scientific, Engitix Therapeutics, Parabilis Medicines Inc. Research Funding: Yes ISCIII, CIBER. Speaking: AstraZeneca, Bayer, BMS, Eli Lilly, Gilead, Roche, Biotoscana Farma. Travel support: Astrazeneca, Roche, Bayer, BMS, Lilly, Ipsen. Principal or sub-Investigator of drug under development: Abbvie, BMS, Adaptimmune, Nerviano, Medivir, Bayer, Ipsen, Astrazeneca, Terumo, Incyte, Roche, Boston Scientific, Medivir. Grant Research Support to the institution: Bayer, Ipsen. Educational Support to the institution: Bayer, Astrazeneca, Eisai- Merck MSD, Roche, Ipsen, Lilly, Terumo, BMS, Next, Boston Scientific, Ciscar Medical, Eventy C3 LLC Egypt. MS-Z: Employment: Hospital Clinic Barcelona. Research Funding: Yes ISCIIII; CIBER. Speaking: Bayer, Roche and AstraZeneca. Travel support: Bayer, BTG, MSD-Eisai and Roche. AF: Employment: Hospital Clinic Barcelona. Consultant or Advisory Role: AstraZeneca, Roche, Boston Scientific. Research Funding: Yes ISCIIII. Speaking: Boston Scientific, Terumo, Roche and AstraZeneca. Travel support: Boston Scientific, AstraZeneca and Roche. NL: Consultant or Advisory Role: Bayer, AstraZeneca, Universal DX. Speaking: Roche, AstraZeneca. Educational Support: Congress fees: Eisai, Travel fees: Bayer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
-
- European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma. J Hepatol. (2025) 82:315–74. doi: 10.1016/j.jhep.2024.08.028 - DOI - PubMed
-
- Fortuny M, García-Calonge M, Arrabal O, Sanduzzi-Zamparelli M, Castano-Garcia A, Gemma Iserte G, et al. Profile of immune-related adverse events and treatment outcomes in first-line immunotherapy for hepatocellular carcinoma. European Association for the Study of the Liver: Liver Cancer Summit; (2024).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

