Transduction of γδ T cells with Baboon envelope pseudotyped lentiviral vector encoding chimeric antigen receptors for translational and clinical applications
- PMID: 40547029
- PMCID: PMC12179110
- DOI: 10.3389/fimmu.2025.1548630
Transduction of γδ T cells with Baboon envelope pseudotyped lentiviral vector encoding chimeric antigen receptors for translational and clinical applications
Abstract
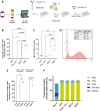
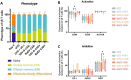
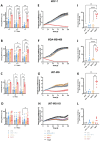
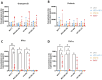
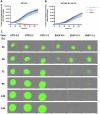
γδ T cells represent a promising cell platform for adoptive cell therapy. Their natural anti-tumor reactivity and HLA-independent target cell recognition make them an attractive platform for allogeneic adoptive immunotherapy clinical interventions. Initial clinical trials exploring allogeneic γδ T-cell therapies have demonstrated encouraging safety profiles. However, their therapeutic efficacy, especially against solid tumors, remains limited. This highlights the need for further optimization of γδ T cell products to improve anti-tumor potency, such as the increased targeting induced by the expression of a chimeric antigen receptors (CAR). However, a critical challenge in the development of CAR-γδ T cell therapies has been optimizing transduction efficiency with standard vector formats allowing for optimal CAR transgene expression that then produces an optimal therapeutic product. Here we present an effective method for enhancing CAR transgene expression in γδ T cells using a Baboon-pseudotyped lentiviral vector (BaEV-LV), comparing it to the conventional vesicular-stomatitis-virus-G protein (VSV-G) LVs. BaEV-LV significantly enhanced the transduction efficiency of γδ T cells with CARs, while conserving the beneficial cell product composition and phenotype of untransduced γδ T cells. The γδ T cells transduced with BaEV-LV CARs demonstrated significantly enhanced cytotoxicity against B7H3-expressing tumor cells in both 2D and 3D in vitro models. Our findings represent a significant advancement in CAR-γδ T cell engineering, offering a promising new avenue for cancer immunotherapy that combines the unique properties of Vγ9Vδ2 T cells with the targeted specificity of CAR technology. This method is compatible with automated closed-system platforms such as the CliniMACS Prodigy®, facilitating Good Manufacturing Practice (GMP)-compliant production for clinical trials. This feature significantly enhances the translational potential of engineered γδ T cells, paving the way for the development of next-generation γδ T cell-based immunotherapies.
Keywords: CAR gd T cells; allogeneic; chimeric antigen receptor; immunotherapy; lentiviral transduction; γδ T cells.
Copyright © 2025 Pinot, Saßor, Möker, Zhang, Verhoeyen, Hidalgo and Orentas.
Conflict of interest statement
Authors LP, AS, NM, CZ, JH, and RO were employed by the company Miltenyi Biotec. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
ADI-270: an armored allogeneic gamma delta T cell therapy designed to target CD70-expressing solid and hematologic malignancies.J Immunother Cancer. 2025 Jul 1;13(7):e011704. doi: 10.1136/jitc-2025-011704. J Immunother Cancer. 2025. PMID: 40592738 Free PMC article.
-
Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors.J Immunother Cancer. 2023 Mar;11(3):e006522. doi: 10.1136/jitc-2022-006522. J Immunother Cancer. 2023. PMID: 36914208 Free PMC article.
-
Current Anti-Myeloma Chimeric Antigen Receptor-T Cells: Novel Targets and Methods.Balkan Med J. 2025 Jul 1;42(4):301-310. doi: 10.4274/balkanmedj.galenos.2025.2025-4-25. Balkan Med J. 2025. PMID: 40619794 Free PMC article. Review.
-
ARI0003: Co-transduced CD19/BCMA dual-targeting CAR-T cells for the treatment of non-Hodgkin lymphoma.Mol Ther. 2025 Jan 8;33(1):317-335. doi: 10.1016/j.ymthe.2024.11.028. Epub 2024 Nov 19. Mol Ther. 2025. PMID: 39563035
-
From spheroids to organoids: next-generation models for CAR-T cell therapy research in solid tumors.Front Immunol. 2025 Jul 11;16:1626369. doi: 10.3389/fimmu.2025.1626369. eCollection 2025. Front Immunol. 2025. PMID: 40718488 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

