Povetacicept (ALPN-303; TACI vTD-Fc), an enhanced, potent dual inhibitor of BAFF and APRIL, ameliorates experimental autoimmune myasthenia gravis in C57BL/6N mice
- PMID: 40547042
- PMCID: PMC12179661
- DOI: 10.3389/fimmu.2025.1533093
Povetacicept (ALPN-303; TACI vTD-Fc), an enhanced, potent dual inhibitor of BAFF and APRIL, ameliorates experimental autoimmune myasthenia gravis in C57BL/6N mice
Abstract
Background and objectives: Myasthenia gravis (MG) is a T cell-dependent, B cell-mediated autoimmune disease targeting the acetylcholine receptor (AChR) and other proteins of the neuromuscular junction postsynaptic membrane. Production of pathogenic autoantibodies results from B cell activation and expansion of antibody-secreting cells, including plasma cells, whose differentiation and survival are reliant on the TNF family cytokines APRIL and BAFF. Povetacicept (ALPN-303; TACI vTD-Fc) is an Fc fusion protein of an engineered TACI domain with significantly more potent dual inhibition of APRIL and BAFF than wild-type (WT) TACI-Fc (e.g., telitacicept).
Methods: In this study, the activity of povetacicept was evaluated in the mouse experimental autoimmune MG (EAMG) model, compared to (i) telitacicept, (ii) a depleting anti-CD20 antibody, (iii) neonatal Fc receptor blocker efgartigimod, (iv) a matched Fc control protein, and (v) PBS as vehicle.
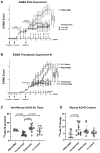
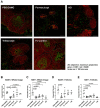
Results: Therapeutic administration of povetacicept ameliorated clinical manifestations in EAMG mice and was associated with significantly lower levels of immunoglobulin subclasses and anti-AChR antibody titers in serum, along with increased muscle AChR content - superior to the evaluated comparators. Povetacicept treatment also reduced the number of total B220+ and Ki67+ proliferating cells in draining lymph node follicles and resulted in modifications of splenic T and B cell subset frequencies, compared to controls.
Discussion: The potent, dual BAFF/APRIL inhibitor povetacicept significantly improves clinical disease activity in EAMG, associated with reductions in pathogenic anti-AChR autoantibodies and superior to comparator therapeutic interventions based on WT TACI-Fc, CD20 depletion, or FcRn inhibition. Povetacicept may therefore confer beneficial clinical outcomes in the treatment of MG and other autoantibody-related neurological diseases.
Keywords: APRIL (TNFSF13); B cell; BAFF - B-cell activating factor; TACI (TNFRSF13B); animal model; experimental autoimmune myasthenia gravis; myasthenia gravis.
Copyright © 2025 Rinaldi, Puleo, Consonni, Miglietti, Mantegazza, Wang, Peng, Lewis, Dillon and Baggi.
Conflict of interest statement
RM has received funding for travel and meeting attendance or advisory board participation from Alexion Pharmaceuticals, Argenx, BioMarin, Catalyst, Sanofi Genzyme, Regeneron Pharmaceuticals and UCB Pharma. Authors NW, SP, KL, and SD were employed by Alpine Immune Sciences, Inc., a Vertex company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that the study received funding from Alpine Immune Sciences, Inc., a Vertex company. The funder had the following involvement in the study: study design, collection, analysis, interpretation of data, and writing of the article.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

