The effect of the EXOPULSE Mollii suit on motor functions in patients with multiple sclerosis - a randomized sham-controlled crossover trial
- PMID: 40547078
- PMCID: PMC12179495
- DOI: 10.1177/20552173251348304
The effect of the EXOPULSE Mollii suit on motor functions in patients with multiple sclerosis - a randomized sham-controlled crossover trial
Abstract
Background: Patients with multiple sclerosis (PwMS) could suffer from frequent and disabling motor symptoms, including balance and mobility problems, spasticity, weakness and fatigue, with an impact on patients' quality of life. Current treatments have limited efficacy or significant side effects. The EXOPULSE Mollii Suit, a transcutaneous electrical nerve stimulation system, provides simultaneous stimulation to 40 muscle groups and may offer a therapeutic alternative.
Objectives: This study evaluated the effects of this device on balance, other motor symptoms and quality of life in PwMS.
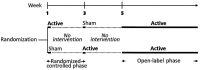
Methods: A randomized, crossover, sham-controlled, double-blind study (phase 1) evaluated the effects of a 60-min single session of active versus sham stimulation. An open-label phase 2 evaluated the effects of stimulation over four weeks. Balance (Berg Balance Scale) was the primary outcome, with secondary measures including spasticity, mobility, pain, fatigue and quality of life.
Results: Thirty-two patients completed phase 1, and 30 completed phase 2. The intervention was well tolerated. Significant improvements in balance (p < 0.001), spasticity (p < 0.001) and fatigue (p = 0.007) were observed in phase 1. Phase 2 showed sustained improvements in balance, spasticity, mobility and quality of life (p < 0.05).
Conclusions: The EXOPULSE Molii Suit demonstrated significant benefits for motor symptoms, warranting further large-scale research into long-term effects.This clinical trial was prospectively registered on clinicaltrials.gov as 'EXOPULSE Mollii Suit, Motor Function & Multiple Sclerosis (EXOSEP)' (NCT06702137). https://clinicaltrials.gov/study/NCT06702137?term=NCT06702137&rank=1.
Keywords: Multiple sclerosis; balance; mobility; spasticity; transcutaneous electric nerve stimulation.
© The Author(s), 2025.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: MC declares having received compensation from Janssen Global Services LLC, Exoneural Network AB, Sweden and Ottobock, France. AC declares having received financial support for research from Biogen, GeNeuro, MedDay, Novartis, Octapharma and Roche and having received honoraria from Biogen, GeNeuro, Novartis and Roche. SA declares having received compensation from Sanofi Aventis, France; Novartis, France; Exoneural Network AB, Sweden and Ottobock, France. The remaining authors declare no conflicts of interest.
Figures

References
-
- Cameron MH, Nilsagard Y. Balance, gait, and falls in multiple sclerosis. Handb Clin Neurol 2018; 159: 237–250. - PubMed
-
- Flachenecker P, Henze T, Zettl UK. Spasticity in patients with multiple sclerosis – clinical characteristics, treatment and quality of life. Acta Neurol Scand 2014; 129: 154–162. - PubMed
-
- Pozzilli C. Overview of MS spasticity. Eur Neurol 2014; 71: 1–3. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

