Construction of cartilaginous organoids based on cartilage extracellular matrix microcarriers to promote articular cartilage regeneration through immune regulation
- PMID: 40547193
- PMCID: PMC12181029
- DOI: 10.1016/j.jot.2025.05.005
Construction of cartilaginous organoids based on cartilage extracellular matrix microcarriers to promote articular cartilage regeneration through immune regulation
Abstract
Objective: To investigate the feasibility of constructing cartilaginous organoids (CORGs) using cartilage extracellular matrix microcarriers (CEMMs), evaluate their ectopic chondrogenic potential, and analyze their impact on in situ repair and regeneration of knee cartilage in SD rats.
Methods: Cartilage extracellular matrix microcarriers (CEMMs) were created through a combination of decellularization, wet milling, and layered sieving methods. The evaluation of their biological function was conducted through live/dead staining, CCK-8 assay, scratch assay, and Transwell assay in a laboratory setting. The immune microenvironment was confirmed to be influenced by CEMMs through a conditioned culture involving rat macrophages. qRT-PCR and secretory function assays was conducted to evaluate the chondrogenic activity of CORGs. Gene expression profiles throughout the development of CORGs were analyzed using transcriptome sequencing. Immunodeficient mouse subcutaneous model to assess the ectopic chondrogenic capacity of CORGs. CORGs were implanted into the knee joint cartilage defects of SD rats to evaluate their effects on cartilage regeneration.
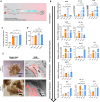
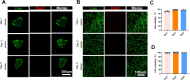
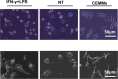
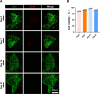
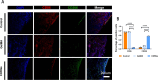
Results: Successfully developed CEMMs with dimensions of 210.4 ± 56.89 um exhibited strong biocompatibility, the capacity to draw in stem cells, stimulate their growth and migration, and encourage macrophages to shift to the M2 type. Functionalized CORGs were successfully constructed based on CEMMs. Transcriptomics showed that CORGs had a gene expression pattern similar to mesodermal to chondrogenic development. CORGs successfully generated cartilaginous tissue subcutaneously in immunodeficient mice. Specifically, at 1 week postoperatively, CORGs were observed to promote M2 polarization of periarticular macrophages. At 6 and 12 weeks post-surgery, gross observation, micro-CT scanning, and histological analyses collectively revealed that CORGs promoted cartilage regeneration.
Conclusions: The functionalized CORGs was successfully constructed based on CEMMs, exhibiting robust expression of chondrogenic-related genes and demonstrating the ability to secrete collagen and GAGs. Transcriptomic analysis revealed that CORGs exhibited a gene expression trajectory consistent with the transition from mesodermal to chondrogenic genes, resulting in the successful development of cartilaginous tissues rich in cartilage-specific matrix when implanted subcutaneously in immunodeficient mice. Furthermore, CORGs demonstrated the ability to modulate the immune microenvironment surrounding the knee joint. In SD rat models of knee cartilage defects, CORGs exhibited robust regenerative and repair capacity.
The translational potential of this article: This research involved the creation of CORGs utilizing natural biomaterials (ECM) and MSCs, demonstrating significant promise for treating cartilage injuries, thereby paving the way for innovative strategies in cartilage tissue regeneration engineering.
Keywords: Cartilage extracellular matrix microcarriers; Cartilage regeneration; Cartilaginous organoids; Immune regulation..
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
















References
LinkOut - more resources
Full Text Sources
Research Materials

