Design, synthesis, and biological evaluation of substituted oxopyridine derivatives as selective FXIa inhibitors
- PMID: 40547257
- PMCID: PMC12177572
- DOI: 10.1039/d4md01013b
Design, synthesis, and biological evaluation of substituted oxopyridine derivatives as selective FXIa inhibitors
Abstract
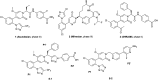
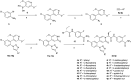
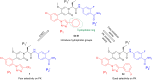
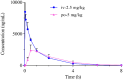
To explore selective FXIa inhibitors, a series of compounds was rationally designed using computer-aided drug design (CADD) and synthesized by introducing hydrophobic P1 fragments, which could strengthen the interaction with the S1 pocket of FXIa and weaken the interaction with plasma kallikrein. All the compounds were tested for the inhibition of FXIa, and some of them were further evaluated via plasma kallikrein selectivity and clotting assays. Compound 4c exhibited excellent in vitro potency, good membrane permeability and higher selectivity than asundexian. Furthermore, 4c showed an excellent pharmacokinetic property in rats after intravenous or oral administration. These results indicate that 4c can serve as a novel FXIa inhibitor that has potential clinical applications in patients.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Design, synthesis and biological evaluation of 6-chloro-quinolin-2-one derivatives as novel FXIa inhibitors.Bioorg Med Chem Lett. 2024 Feb 1;99:129610. doi: 10.1016/j.bmcl.2024.129610. Epub 2024 Jan 9. Bioorg Med Chem Lett. 2024. PMID: 38211702
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.Health Technol Assess. 2006 Sep;10(31):iii-iv, xiii-xvi, 1-239. doi: 10.3310/hta10310. Health Technol Assess. 2006. PMID: 16948890
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
References
-
- Xie Z. L. Meng Z. W. Yang X. X. Duan Y. J. Wang Q. Liao C. Z. J. Med. Chem. 2023;66:5332–5363. - PubMed
-
- Wells P. S. Forgie M. A. Rodger M. A. JAMA. 2014;311:717–728. - PubMed
-
- Rupprecht H. Blank R. Drugs. 2010;70:2153–2170. - PubMed
-
- Perzborn E. Roehrig S. Straub A. Kubitza D. Misselwitz F. Nat. Rev. Drug Discovery. 2011;10:61–75. - PubMed
-
- Di Nisio M. Middeldorp S. Büller H. R. N. Engl. J. Med. 2005;353:1028–1040. - PubMed
LinkOut - more resources
Full Text Sources

