Risk factors analysis and nomogram development for myelosuppression in diffuse large B-cell lymphoma patients undergoing first-line chemotherapy: a dual-centre retrospective cohort study
- PMID: 40547311
- PMCID: PMC12180452
- DOI: 10.7717/peerj.19539
Risk factors analysis and nomogram development for myelosuppression in diffuse large B-cell lymphoma patients undergoing first-line chemotherapy: a dual-centre retrospective cohort study
Abstract
Objective: The primary objective of this research was to examine the characteristics of myelosuppression following first-line chemotherapy in patients suffering from diffuse large B-cell lymphoma (DLBCL). Furthermore, the study aimed to identify and analyze the risk factors impacting myelosuppression after chemotherapy and to construct a predictive model for evaluating the risk of myelosuppression.
Methods: This retrospective cohort study was conducted across two medical centers. The study included 243 patients with DLBCL treated at the Anning First People's Hospital Affiliated with Kunming University of Science and Technology from January 2022 to December 2023 as the development cohort, and 107 patients treated at the Third Affiliated Hospital of Kunming Medical University from January 2024 to May 2024 as the validation cohort. The study investigated the incidence of myelosuppression in all patients, identified independent factors influencing this condition through logistic regression analysis, and constructed and validated a nomogram. Finally, the model's performance was evaluated using both internal and external validation cohorts.
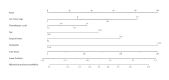
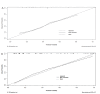
Results: The research rigorously incorporated a cohort of 243 DLBCL patients, with myelosuppression observed in 93 individuals (38.27%). Multifactorial analysis revealed that the chemotherapy cycle, age, Ann Arbor stage, surgical history, and neutrophil levels were independently correlated with myelosuppression following initial chemotherapy in DLBCL patients. A nomogram was developed based on the multifactorial analysis. The receiver operating characteristic (ROC) analysis revealed myelosuppression in the nomogram of both the development set (area under the curve (AUC = 0.834, 95% CI [0.785-0.884]) and the validation set (AUC = 0.861, 95% CI [0.791-0.931])), indicating clear differentiation. Further calibration curve analysis and decision curve analysis (DCA) revealed strong calibration and clinical utility of the column-line graph model.
Conclusion: Patients with DLBCL are at an increased risk and frequency of myelosuppression following first-line chemotherapy. The development of a highly accurate prediction model for myelosuppression in this patient population facilitates individualized treatment strategies. Future studies should focus on expanding the sample size and developing and validating the model in additional types of cancer.
Keywords: Forecasting; Myelosuppression; Nomograms; Non-Hodgkin lymphoma; Risk.
© 2025 Wang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Berehab M, Rouas R, Akl H, Duvillier H, Journe F, Fayyad-Kazan H, Ghanem G, Bron D, Lewalle P, Merimi M. Apoptotic and non-apoptotic modalities of thymoquinone-induced lymphoma cell death: highlight of the role of cytosolic calcium and necroptosis. Cancers (Basel) 2021;13(14):3579. doi: 10.3390/cancers13143579. - DOI - PMC - PubMed
-
- Bishnoi R, Bennett J, Reisman DN. Palliative treatment of patients with inoperable locally advanced, recurrent or metastatic head and neck squamous cell cancer, using a low-dose and personalized chemotherapeutic regimen. Oncology Letters. 2017;13(6):4633–4640. doi: 10.3892/ol.2017.6068. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

