One-step strategy for fabricating icariin-encapsulated biomimetic Scaffold: Orchestrating immune, angiogenic, and osteogenic cascade for enhanced bone regeneration
- PMID: 40547322
- PMCID: PMC12182316
- DOI: 10.1016/j.bioactmat.2025.06.001
One-step strategy for fabricating icariin-encapsulated biomimetic Scaffold: Orchestrating immune, angiogenic, and osteogenic cascade for enhanced bone regeneration
Abstract
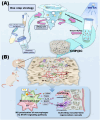
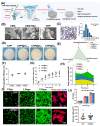
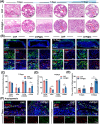
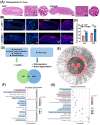
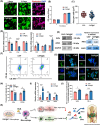
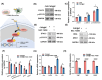
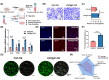
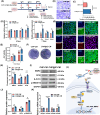
The repair of bone defects relies on the intricate coordination of inflammation, angiogenesis, and osteogenesis. However, scaffolds capable of integrating osteo-immunomodulation and vascular-bone coupling to cascade-activate these processes remain a challenge. Here, a biomimetic scaffold (CHP@IC) with in situ PLGA@icariin (PLGA@IC) microspheres encapsulation was successfully fabricated using a one-step emulsification and polymerization strategy. This approach not only simplifies the fabrication process but also ensures high encapsulation efficiency and sustained release of IC through PLGA@IC microspheres. The findings from subcutaneous implantation, network pharmacology-predicted molecular targets, and in vitro studies collectively reveal that the CHP@IC-induced M2 polarization of macrophages via STAT3 signaling pathway triggers the sequential activation of inflammation, angiogenesis, and osteogenesis to enhance bone regeneration. The CHP@IC scaffold exhibited a significant osteogenic advantage in cranial defect repair, yielding new bone volumes approximately 3-fold and 10-fold greater than those in the CHP group and blank control group, respectively. This study not only elucidates the mechanism of IC in promoting regeneration of bone but also provides a novel method for designing scaffolds aimed at the efficient repair of bone defects.
Keywords: Biomimetic scaffold; Bone regeneration; Cascade activation; Icariin delivery; One-step strategy.
© 2025 The Authors.
Conflict of interest statement
Kai Zhang is an editorial board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Figures

Similar articles
-
Oncostatin-M functionalized cryogel microspheres for promoting diabetic bone defects regeneration.J Orthop Translat. 2025 Jun 20;53:138-148. doi: 10.1016/j.jot.2025.06.002. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40606844 Free PMC article.
-
Microsphere-Mediated Sustained Delivery of Growth Factors Stimulates Osteogenesis in Target Cells in a Three-Dimensional Microenvironment.J Biomed Mater Res A. 2025 Jun;113(6):e37947. doi: 10.1002/jbm.a.37947. J Biomed Mater Res A. 2025. PMID: 40499148
-
Injectable hydrogel scaffold incorporating microspheres containing cobalt-doped bioactive glass for bone healing.J Biomed Mater Res A. 2024 Dec;112(12):2225-2242. doi: 10.1002/jbm.a.37773. Epub 2024 Jul 10. J Biomed Mater Res A. 2024. PMID: 38984402
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
-
- Xue X., Hu Y., Deng Y., Su J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021;31(19) doi: 10.1002/adfm.202009432. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

