Exploring Providers' Behaviors, Attitudes, and Preferences on the Treatment of Pulmonary Arterial Hypertension With Endothelin Receptor Antagonist (ERA) + Phosphodiesterase-5 Inhibitors (PDE5i)
- PMID: 40547449
- PMCID: PMC12182709
- DOI: 10.1002/pul2.70113
Exploring Providers' Behaviors, Attitudes, and Preferences on the Treatment of Pulmonary Arterial Hypertension With Endothelin Receptor Antagonist (ERA) + Phosphodiesterase-5 Inhibitors (PDE5i)
Abstract
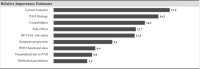
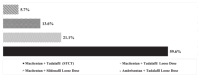
This study aims to understand healthcare providers' (HCPs) decision to adopt double combination therapy with ERA + PDE5i for pulmonary arterial hypertension (PAH), and to explore whether a single tablet combination therapy (STCT) might increase adoption practices. 195 US HCPs completed a survey evaluating their PAH treatment preferences. HCPs' willingness to use double combination ERA + PDE5i was assessed using a discrete choice experiment (DCE). The sample predominantly included physicians (73.3%) from centers of excellence (63.1%), with a mean of 117.4 PAH patients treated in the past year. Key factors influencing ERA + PDE5i adoption in the DCE were the patient's current treatment (17.9), PAH etiology (16.2), existing comorbidities (14.1), and history of side effects (12.7), with higher scores indicating stronger preference. HCPs were more likely to select ERA + PDE5i for patients currently on PDE5i monotherapy, with idiopathic PAH, and without comorbidities or a history of side effects. Regarding STCT, most HCPs reported that it would allow them to initiate ERA + PDE5i sooner (76.4%) and improve patient compliance (82.6%). However, concerns regarding cost/insurance issues (63.6%) and a history of side effects (50.8%) were identified as limitations to adopting STCT. Patients' current therapy, the cause of PAH, comorbidities, and side effects are key factors influencing whether US providers are willing to treat them with ERA + PDE5i. Providers perceive that STCT may help HCPs initiate ERA + PDE5i sooner, improve compliance, and simplify initiation of upfront double therapy and delivery of triple therapy. Addressing cost and insurance barriers will be critical to realizing these potential benefits.
Keywords: ERA; PDE5i; combination therapy; pulmonary arterial hypertension; treatment preferences.
© 2025 The Author(s). Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
Gabriela Gomez Rendon, David Lopez, Carly J. Paoli, Mohammad Rahman: Employees of Johnson & Johnson (J&J) and hold stock in J&J. Abraham Lee, and November McGarvey: Were employees of BluePath Solutions (BPS) at time of research but are no longer employed by BPS. Nicholas A. Kolaitis: Has received financial support from J&J, Merck, Liquidia, United Therapeutics, Bayer, and Gossamer. Melisa Wilson: Has received financial support from J&J, Merck, Bayer, United Therapeutics. Martha Kingman: Has received financial support from Aerami, Merck, J&J, Liquidia, Gossamer. Lana Melendres‐Groves: Has received financial support from J&J, United Therapeutic, Bayer, Merck.
Figures


Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.Health Technol Assess. 2005 Apr;9(15):1-157, iii-iv. doi: 10.3310/hta9150. Health Technol Assess. 2005. PMID: 15842952
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Galiè N., Humbert M., Vachiery J. L., et al., “2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT),” European Heart Journal 37 (2016): 67–119, 10.1093/eurheartj/ehv317. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

