Flow-induced mechano-modulation of intestinal permeability on chip
- PMID: 40547488
- PMCID: PMC12180969
- DOI: 10.1016/j.mtbio.2025.101951
Flow-induced mechano-modulation of intestinal permeability on chip
Abstract
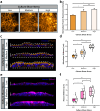
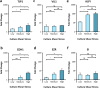
The comprehension of the working principles behind the intestinal transepithelial transport is critical in nutrient and drug development research. Within this framework, microfluidic microphysiological platforms are on the verge of overshadowing traditional in vitro systems due to their accuracy in replicating key physiological features of the native tissue. Nevertheless, the effects of fluid mechanical stimuli on the selective permeation characteristics of the gut barrier are still unexplored. This is an indispensable feature for designing more biorelevant organ-on-chip models. Here, an intestine-on-chip platform is conceived to mechanically stimulate cells with three different fluid shear stresses and investigate the relative flow-induced changes of molecule transport alongside the resulting epithelial architecture and barrier functionality. Our results reveal that epithelia grown at lower shears exhibit a ∼1.5 higher and faster paracellular permeability while showing a ∼3 times lower and delayed transcellular uptake compared to layers exposed to higher shear stress. This is corroborated by impedance spectroscopy measurements that display altered tight junctional and bilayer resistance, as well as an increased capacitance of the epithelium in response to variations in mechanical stress within the culture. Taken together, these findings advocate that fluid shear stress can serve as mechano-modulator not only for intestinal transport but also for other epithelial cell lines under physiological circumstances.
Keywords: Gut-on-chip; Microfluidics; Permeability; Shear stress; TEER.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Pluske J.R., Thompson M.J., Atwood C.S., Bird P.H., Williams I.H., Hartmann P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows' whole milk after weaning. Br. J. Nutr. Sep. 1996;76(3):409–422. doi: 10.1079/BJN19960046. - DOI - PubMed
LinkOut - more resources
Full Text Sources

