Electron Transfer Mechanism from the Oxygen-Evolving Complex to the Electron-Acceptor Tyrosine during the S2 to S3 Transition in Photosystem II
- PMID: 40547619
- PMCID: PMC12177751
- DOI: 10.1021/acsomega.5c00945
Electron Transfer Mechanism from the Oxygen-Evolving Complex to the Electron-Acceptor Tyrosine during the S2 to S3 Transition in Photosystem II
Abstract
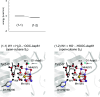
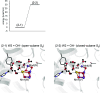
The mechanism of water oxidation catalyzed by Mn4CaO5 in photosystem II lacks consensus, particularly regarding proton-coupled electron transfer in the second flash-induced S2 to S3 transition. Here, we investigate the electron transfer mechanism during the S2 to S3 transition using a quantum mechanical/molecular mechanical/polarizable continuum model approach. The electrostatic interaction with the oxidized redox-active tyrosine, TyrZ, triggers proton release not from a ligand water molecule near chloride (W2) at dangling Mn (Mn4) or a ligand water molecule at Ca2+ (W3), but from a ligand water molecule (W1) near D1-Asp61 at Mn4, forming OH- at Mn-(IV). OH- formation at Ca2+ is significantly less stable than that at Mn4-(IV). Incorporation of the OH- species into Mn4CaO5 induces a valence-state conversion from (III,IV,IV,IV) to (IV,IV,IV,III). Interestingly, subsequent water incorporation from a water channel and restoration of the H-bond network of TyrZ not only elevate the redox potential of TyrZ but also convert the valence state back to (III,IV,IV,IV), facilitating electron transfer to TyrZ. The electronic coupling between Mn1 and TyrZ is 1 to 3 meV in the S2 to S3 transition, significantly smaller than those between Mn4 and TyrZ in the S0 to S1 (∼170 meV) and S1 to S2 (∼120 meV) transitions. This step serves as the rate-limiting step if [Mn-(IV)]4 is considered to be the relevant state to S3.
© 2025 The Authors. Published by American Chemical Society.
Figures




Similar articles
-
Electron-Transfer Route in the Early Oxidation States of the Mn4CaO5 Cluster in Photosystem II.J Phys Chem B. 2023 Jan 12;127(1):205-211. doi: 10.1021/acs.jpcb.2c08246. Epub 2022 Dec 21. J Phys Chem B. 2023. PMID: 36542840 Free PMC article.
-
Current perspectives on proton and electron transfer pathways in photosystem II water oxidation.Plant Cell Physiol. 2025 Aug 30:pcaf107. doi: 10.1093/pcp/pcaf107. Online ahead of print. Plant Cell Physiol. 2025. PMID: 40884490
-
Interplay of two low-barrier hydrogen bonds in long-distance proton-coupled electron transfer for water oxidation.PNAS Nexus. 2023 Dec 7;2(12):pgad423. doi: 10.1093/pnasnexus/pgad423. eCollection 2023 Dec. PNAS Nexus. 2023. PMID: 38130665 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Diner B. A., Schlodder E., Nixon P. J., Coleman W. J., Rappaport F., Lavergne J., Vermaas W. F. J., Chisholm D. A.. Site-directed mutations at D1-His198 and D2-His197 of photosystem II in Synechocystis PCC 6803: sites of primary charge separation and cation and triplet stabilization. Biochemistry. 2001;40:9265–9281. doi: 10.1021/bi010121r. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
