Chalcogen Derivatives for the Treatment of African Trypanosomiasis: Biological Evaluation of Thio- and Seleno-Semicarbazones and Their Azole Derivatives
- PMID: 40547650
- PMCID: PMC12177781
- DOI: 10.1021/acsomega.5c02014
Chalcogen Derivatives for the Treatment of African Trypanosomiasis: Biological Evaluation of Thio- and Seleno-Semicarbazones and Their Azole Derivatives
Abstract
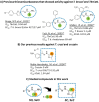
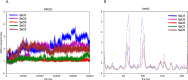
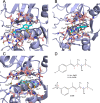
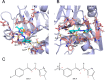
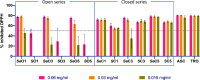
Human African trypanosomiasis (HAT) is caused by Trypanosoma brucei. Drug therapy remains challenging due to drug resistance and/or toxicity. New drugs are needed. Using thiosemicarbazones as a starting point, we employed a S to Se isosteric replacement strategy to design 44 analogs which were evaluated against T. brucei in vitro. Compounds were divided into 11 groups of four derivatives corresponding to thio-, selenosemicarbazones, and their cyclic counterparts, thio- and selenazoles. We selected three groups which contained a total of six derivatives that inhibited parasite growth by >70%. Then, we investigated the mechanism of action of these compounds, performing quantitative assays to measure their inhibition of the T. brucei cathepsin L-like protease (TbrCATL) and DPPH antioxidant activities. The lead compound (SeO3) showed antioxidant capacity and the best activity against T. brucei (EC50 = 0.47 μM). Nevertheless, its toxicity should be improved. We also predicted the interactions of these compounds with TbrCATL utilizing molecular dynamics. We demonstrate that the Se derivatives are more active than their S analogues, and that the selenazole ring decreases Se-associated toxicity. Also, thio- and selenosemicarbazones are more potent against TbrCATL than the cyclic derivatives. We conclude that TbrCATL inhibition should be combined with antioxidant activity to obtain active compounds against T. brucei.
© 2025 The Authors. Published by American Chemical Society.
Figures

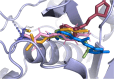
References
-
- World Health Organization Trypanosomiasis, human African (sleeping sickness). https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-a... (accessed 2024, August 1).
-
- DNDI Sleeping sickness: Symptoms, transmission, and current treatments for sleeping sickness. https://dndi.org/diseases/sleeping-sickness/facts/. (accessed 2024, August 1).
-
- Coetzer, J. A. W. ; Thomson, G. R. ; Tustin, R. C. . Infectious diseases of livestock with special reference to Southern Africa; Oxford University Press: Cape Town, 1994.
-
- Pereira G. A., Santos L. H., Wang S. C., Martins L. C., Villela F. S., Liao W., Dessoy M. A., Dias L. C., Andricopulo A. D., Costa M. A.. et al. Benzimidazole inhibitors of the major cysteine protease of Trypanosoma brucei. Future Med. Chem. 2019;11:1537–1551. doi: 10.4155/fmc-2018-0523. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
