Effects of Apilarnil on Type 2 Diabetes-Induced IRS-1/PI3K/Akt Mediated Insulin Resistance in Male Rats
- PMID: 40547654
- PMCID: PMC12177753
- DOI: 10.1021/acsomega.5c02662
Effects of Apilarnil on Type 2 Diabetes-Induced IRS-1/PI3K/Akt Mediated Insulin Resistance in Male Rats
Abstract
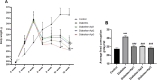
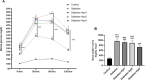
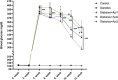
Diabetes is one of the most important chronic and metabolic health problems seen as an epidemic of the 21st century. The incidence of diabetes is gradually increasing, and different methods are used to treat it. Apitherapy products such as honey, propolis, royal jelly, and bee venom can be used as an alternative to treat diabetes and reduce symptoms. One of these apitherapy products is Apilarnil, which has not yet become popular. The hypolipidemic, hepatoprotective, androgenic, anabolic, and immune system enhancing properties of Apilarnil indicate that it may be effective in the treatment of type 2 diabetes or in reducing the symptoms. The study aimed to determine the therapeutic effect of Apilarnil on insulin resistance and the possible underlying mechanism. In the study, 40 8 week-old male Wistar albino rats were used. The rats were divided into 5 groups as Control, Diabetes, Diabetes + Api1 (125 mg/kg/day lyophilized Apilarnil), Diabetes + Api2 (250 mg/kg/day lyophilized Apilarnil) and Diabetes + Api3 (500 mg/kg/day lyophilized Apilarnil). Each group consisted of 8 rats. Apilarnil doses were administered by oral gavage with 1 mL of distilled water, 5 days in a week, during the last 6 weeks. The levels of Nrf2, NF-κB, TNF-α, total IRS-1, p-IRS-1, PI3K p85, total Akt, p-Akt, IL-1B, and GLUT4 proteins were determined in target tissues by Western blotting. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC), and triglyceride (TG) levels were detected in the serum. Serum superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), and insulin levels were determined by the ELISA method. According to the data obtained in the study, the application of Apilarnil, compared to the diabetes group, balanced some findings, but did not demonstrate a fully antidiabetic effect.
© 2025 The Authors. Published by American Chemical Society.
Figures

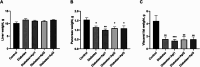




Similar articles
-
Therapeutic effects of apilarnil, a bee product, on apoptosis and autophagy in cisplatin-induced rat ovarian toxicity.Food Chem Toxicol. 2025 Sep;203:115594. doi: 10.1016/j.fct.2025.115594. Epub 2025 Jun 18. Food Chem Toxicol. 2025. PMID: 40541689
-
Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats.J Ethnopharmacol. 2017 Mar 6;199:119-127. doi: 10.1016/j.jep.2017.02.003. Epub 2017 Feb 2. J Ethnopharmacol. 2017. PMID: 28163112
-
Psychological and/or educational interventions for the prevention of depression in children and adolescents.Cochrane Database Syst Rev. 2004;(1):CD003380. doi: 10.1002/14651858.CD003380.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2011 Dec 07;(12):CD003380. doi: 10.1002/14651858.CD003380.pub3. PMID: 14974014 Updated.
-
[Evaluation of the combined effect of Arthrospira platensis biomass phycyanin concentrate and soy protein on male Wistar rats fed a high-fat diet with added cholesterol].Vopr Pitan. 2025;94(2):73-84. doi: 10.33029/0042-8833-2025-94-2-73-84. Epub 2025 Mar 19. Vopr Pitan. 2025. PMID: 40418136 Russian.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- Kiziltas H., Bingol Z., Goren A. C., Pinar S. M., Ortaakarsu A. B., Alwasel S. H., Gulcin I. ˙.. Comprehensive metabolic profiling of acantholimon caryophyllaceum using LC–HRMS and evaluation of antioxidant activities, enzyme inhibition properties and molecular docking studies. S. Afr. J. Bot. 2022;151:743–755. doi: 10.1016/j.sajb.2022.10.048. - DOI
-
- Mutlu M., Bingol Z., Uc E. M., Köksal E., Goren A. C., Alwasel S. H., Gulcin I. ˙.. Comprehensive metabolite profiling of cinnamon (cinnamomum zeylanicum) leaf oil using LC-HR/MS, GC/MS, and GC-FID: Determination of antiglaucoma, antioxidant, anticholinergic, and antidiabetic profiles. Life. 2023;13(1):136. doi: 10.3390/life13010136. - DOI - PMC - PubMed
-
- Miaffo D., Ntchapda F., Mahamad T. A., Maidadi B., Kamanyi A.. Hypoglycemic, antidyslipidemic and antioxydant effects of vitellaria paradoxa barks extract on high-fat diet and streptozotocin-induced type 2 diabetes rats. Metab. Open. 2021;9:100071. doi: 10.1016/j.metop.2020.100071. - DOI - PMC - PubMed
-
- Li J. Q., Welchowski T., Schmid M., Letow J., Wolpers C., Pascual-Camps I., Holz F. G., Finger R. P.. Prevalence, incidence and future projection of diabetic eye disease in europe: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020;35:11–23. doi: 10.1007/s10654-019-00560-z. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
