Trans-Cyclooctene Isomerization Catalyzed by Thiamine Degradation Products in Cell Culture Media
- PMID: 40547704
- PMCID: PMC12177633
- DOI: 10.1021/acsomega.5c01780
Trans-Cyclooctene Isomerization Catalyzed by Thiamine Degradation Products in Cell Culture Media
Abstract
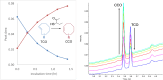
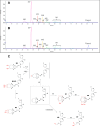
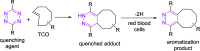
PET imaging of intrathecally dosed ASO neurology drugs is challenging due to the long time needed to achieve steady-state brain distribution, making the use of a simple 18F tag impossible with its short radioactive half-life. To overcome this challenge, a pretargeted imaging solution has been developed in which an ASO tagged with a tetrazine is dosed intrathecally, and after 24 h a reactive trans-cyclooctene (TCO) tagged with 18F is dosed intravenously. The two molecules form a click-chemistry adduct, allowing for PET imaging scans immediately following 18F-TCO administration. Although it has been demonstrated that TCOs can be relatively stable in vivo, they rapidly isomerize to cis-cyclooctenes (CCOs) in cell culture media and "aged" plasma, making many DMPK experiments challenging to interpret and not representative of the in vivo stability. The predominant cause of isomerization was determined to be thiamine degradation product(s) in media such as DMEM. Several techniques to overcome the challenges of in vitro and ex vivo isomerization during analytical experiments are herein proposed, such as the use of custom media and/or fresh plasma, adding antioxidants, using surrogate molecules, and using TCO trapping agents. These findings and techniques may also be relevant to other applications in which TCOs are incubated in thiamine-containing cell culture media.
© 2025 The Authors. Published by American Chemical Society.
Figures
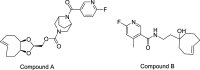



Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
-
Thrombolysis for acute ischaemic stroke.Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD000213. doi: 10.1002/14651858.CD000213.pub2. PMID: 12917889 Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
LinkOut - more resources
Full Text Sources
