Diffusion tensor imaging of sequential neuropathological patterns in progressive supranuclear palsy
- PMID: 40547840
- PMCID: PMC12179216
- DOI: 10.3389/fnagi.2025.1569302
Diffusion tensor imaging of sequential neuropathological patterns in progressive supranuclear palsy
Abstract
Background and objective: A neuropathological cerebral staging concept for progressive supranuclear palsy (PSP) has been proposed that tau inclusions in PSP may progress in a sequential regional pattern. The objective was to develop a hypothesis-guided region/tract of interest-based (ROI/TOI) approach to use diffusion tensor imaging (DTI) targeted to analyze in vivo the regions that are prone to be involved at each neuropathological stage of PSP.
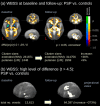
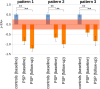
Methods: Two data cohorts were analyzed: cohort A of 78 PSP patients [55 Richardson's syndrome (PSP-RS) and 23 PSP with predominant parkinsonism (PSP-P)] and 63 controls, recorded at 3.0T at multiple sites, and a single-site cohort B constituted by 1.5T data of 66 PSP patients (46 PSP-RS and 20 PSP-P) and 44 controls. In cohort A, 21 PSP patients (13 PSP-RS and 8 PSP-P) and 17 controls obtained a follow-up scan after 17 months. Whole brain-based spatial statistics (WBSS) was used to identify the alterations in PSP patients vs. controls. The combined ROI- and TOI-based approach targeted structures that are prone to be involved during the course of PSP.
Results: WBSS demonstrated alterations predominantly in brainstem/midbrain, basal ganglia, and frontal lobe, more pronounced in the longitudinal data. Statistical analyses of the ROIs/TOIs showed a sequential pattern of structures that were assigned to previously defined neuropathological steps.
Conclusion: The combined ROI- and TOI-based DTI approach was able to map the disease stages of PSP in vivo cross-sectionally and longitudinally, lending support to DTI as a technical marker for imaging disease progression according to PSP stages. This approach might be useful as a tool for stratification of PSP patients MRI with respect to its proposed neuropathological progression in future longitudinal and autopsy-controlled studies.
Keywords: diffusion tensor imaging (DTI); neuropathology; progressive supranuclear palsy; sequential pattern; tau protein.
Copyright © 2025 Bârlescu, Höglinger, Volkmann, Ludolph, Del Tredici, Braak, Müller and Kassubek.
Conflict of interest statement
Günter Höglinger has ongoing research collaborations with Roche, UCB, Abbvie; serves as a consultant for Abbvie, Alzprotect, Amylyx, Aprinoia, Asceneuron, Bayer, Bial, Biogen, Biohaven, Epidarex, Ferrer, Kyowa Kirin, Lundbeck, Novartis, Retrotope, Roche, Sanofi, Servier, Takeda, Teva, UCB; received honoraria for scientific presentations from Abbvie, Bayer, Bial, Biogen, Bristol Myers Squibb, Esteve, Kyowa Kirin, Pfizer, Roche, Teva, UCB, Zambon. holds a patent “Treatment of Synucleinopathies” United States Patent No. US 10,918,628 B2/European Patent Patent No.: EP 17 787 904.6-1109/3 525 788; received publication royalties from Academic Press, Kohlhammer, and Thieme. Günter Höglinger was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198); European Joint Programme on Rare Diseases (Improve-PSP); Petermax-Müller Foundation (Etiology and Therapy of Synucleinopathies and Tauopathies). Jan Kassubek has received honoraria or consultation fees from AbbVie, Bial, Biogen, Desitin, Esteve, Licher MT, Medtronic, NeuroDerm, Novartis, STADA, UCB Pharma, and Zambon; in addition, he is Specialty Chief Editor for Frontiers in Neurology (section Applied Neuroimaging) and Associate Editor (Neurology) for Therapeutic Advances in Chronic Disease. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Brain Microstructure Interrogation by Diffusion Tensor and Kurtosis Imaging in Progressive Supranuclear Palsy Subtypes.J Neuroimaging. 2025 May-Jun;35(3):e70062. doi: 10.1111/jon.70062. J Neuroimaging. 2025. PMID: 40556376
-
Cerebral Tau Deposition in Comorbid Progressive Supranuclear Palsy and Amyotrophic Lateral Sclerosis: An [18F]-Flortaucipir and 7T MRI Study.Neurodegener Dis. 2023;23(3-4):35-42. doi: 10.1159/000536614. Epub 2024 Mar 25. Neurodegener Dis. 2023. PMID: 38527450 Free PMC article.
-
Associations between neuropsychological profile and regional brain FDG uptake in progressive supranuclear palsy.J Parkinsons Dis. 2025 Jun;15(4):904-912. doi: 10.1177/1877718X251343080. Epub 2025 May 25. J Parkinsons Dis. 2025. PMID: 40415458
-
Effectiveness of allied health therapy in the symptomatic management of progressive supranuclear palsy: a systematic review.JBI Database System Rev Implement Rep. 2016 Jun;14(6):148-95. doi: 10.11124/JBISRIR-2016-2002352. JBI Database System Rev Implement Rep. 2016. PMID: 27532657
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Albrecht F., Bisenius S., Neumann J., Whitwell J., Schroeter M. (2019). Atrophy in midbrain & cerebral/cerebellar pedunculi is characteristic for progressive supranuclear palsy - A double-validation whole-brain meta-analysis. Neuroimage Clin. 22:101722. 10.1016/j.nicl.2019.101722 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

