Insights into retention and safety/tolerability of apomorphine sublingual film in patients with Parkinson's disease and OFF episodes: post hoc analyses of a phase III, open-label study
- PMID: 40547865
- PMCID: PMC12181698
- DOI: 10.1177/17562864251343583
Insights into retention and safety/tolerability of apomorphine sublingual film in patients with Parkinson's disease and OFF episodes: post hoc analyses of a phase III, open-label study
Abstract
Background: Managing OFF episodes in patients with Parkinson's disease becomes increasingly challenging over time, making it critical to tailor treatment to each patient's needs and characteristics for effective care.
Objectives: Study CTH-301 assessed the long-term safety/tolerability and efficacy of sublingual apomorphine (SL-APO) for the on-demand treatment of OFF episodes.
Design: The findings from four post hoc analyses of Study CTH-301, conducted to understand factors influencing SL-APO retention and safety/tolerability, with a particular focus on oropharyngeal treatment-emergent adverse events (TEAEs) are reported.
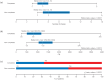
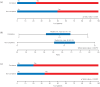
Methods: The first analysis evaluated baseline variables differing between patients who completed the study and those who discontinued due to either lack of efficacy or adverse events to help define patients more likely to benefit from SL-APO therapy: The second and third analyses compared safety/tolerability between the subgroups of patients who were or were not receiving dopamine agonist (DA) treatment, and in those aged <70 or ⩾70 years at baseline, respectively. The fourth analysis examined oropharyngeal TEAEs.
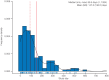
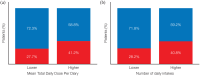
Results: Patients in a younger age group, those experiencing morning akinesia or delayed ON, and those taking lower dose/fewer intakes of levodopa and concomitant DAs were more likely to benefit from SL-APO therapy. Patients taking concomitant DAs reported lower rates of DA-related TEAEs and a higher mean SL-APO optimal dose. Specific analyses in patients aged ⩾70 years indicated that this age group reported similar rates of TEAEs and a similar profile of the most common TEAEs compared with the group aged <70 years. A lower total daily dose of SL-APO was associated with a reduced risk of developing oropharyngeal TEAEs. Such events were mostly mild or moderate, occurring within the first months after SL-APO initiation, and generally resolved, with worsening being rare.
Conclusion: These analyses provided insights into retention and safety/tolerability of SL-APO, helping clinicians and patients make informed treatment decisions.
Keywords: OFF episodes; Parkinson’s disease; adverse events; dopamine agonist; post hoc analysis; sublingual apomorphine.
© The Author(s), 2025.
Figures
Similar articles
-
Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD012200. doi: 10.1002/14651858.CD012200.pub2. Cochrane Database Syst Rev. 2017. PMID: 28440858 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
The Long-Term Impact of Levodopa/Carbidopa Intestinal Gel on 'Off'-time in Patients with Advanced Parkinson's Disease: A Systematic Review.Adv Ther. 2021 Jun;38(6):2854-2890. doi: 10.1007/s12325-021-01747-1. Epub 2021 May 20. Adv Ther. 2021. PMID: 34018146 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD013198. doi: 10.1002/14651858.CD013198.pub2. Cochrane Database Syst Rev. 2021. PMID: 33448349 Free PMC article.
References
-
- Olanow CW, Factor SA, Espay AJ, et al. Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol 2020; 19: 135–144. - PubMed
-
- Bial Pharma UK Ltd. KYNMOBI™ (apomorphine hydrochloride) summary of product characteristics, https://wwwmedicinesorguk/emc/product/14890/smpc#about-medicine (2023, April 2025).
-
- Kassubek J, Stocchi F, Balaguer E, et al. Feasibility of home dose optimization of apomorphine sublingual film in Parkinson’s disease patients with OFF episodes: results from the dose-optimization phase of an open-label, randomized crossover study. Ther Adv Neurol Disord 2023; 16: 17562864231209240. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

