Nitric oxide is not responsible for initial sensory-induced neurovascular coupling response in the barrel cortex of lightly anesthetized mice
- PMID: 40548064
- PMCID: PMC12180670
- DOI: 10.1117/1.NPh.12.S2.S22802
Nitric oxide is not responsible for initial sensory-induced neurovascular coupling response in the barrel cortex of lightly anesthetized mice
Abstract
Significance: Neurovascular coupling matches changes in neural activity to localized changes in cerebral blood flow. Although much is known about the role of excitatory neurons in neurovascular coupling, that of inhibitory interneurons is unresolved. Although neuronal nitric oxide synthase (nNOS)-expressing interneurons are capable of eliciting vasodilation, the role of nitric oxide in neurovascular coupling is debated.
Aim: We investigated the role of nitric oxide in hemodynamic responses evoked by nNOS-expressing interneurons and whisker stimulation in mouse sensory cortex.
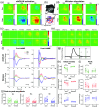
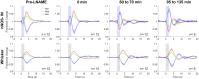
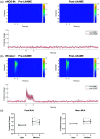
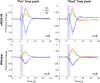
Approach: In lightly anesthetized mice expressing channelrhodopsin-2 in nNOS-interneurons, 2D optical imaging spectroscopy was applied to measure stimulation-evoked cortical hemodynamic responses. To investigate the underlying vasodilatory pathways involved, the effects of pharmacological inhibitors of NOS and 20-HETE were assessed.
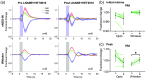
Results: Hemodynamic responses evoked by nNOS-expressing interneurons were altered in the presence of the NOS inhibitor LNAME, revealing an initial 20-HETE-dependent vasoconstriction. By contrast, the initial sensory-evoked hemodynamic response was largely unchanged.
Conclusions: Our results challenge the involvement of nNOS-expressing interneurons and nitric oxide in the initiation of functional hyperemia, suggesting that nitric oxide may be involved in the recovery, rather than initiation, of sensory-induced hemodynamic responses.
Keywords: inhibitory interneuron; neuronal nitric oxide synthase interneuron; neurovascular coupling; nitric oxide; optogenetics; vasomotion.
© 2025 The Authors.
Figures



References
Associated data
LinkOut - more resources
Full Text Sources

