Augmented use of L-asparaginase markedly improves AYA ALL outcomes: FBMTG prospective MRD2014 study
- PMID: 40548197
- PMCID: PMC12182846
- DOI: 10.1016/j.bneo.2024.100033
Augmented use of L-asparaginase markedly improves AYA ALL outcomes: FBMTG prospective MRD2014 study
Abstract
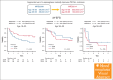
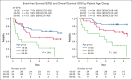
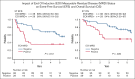
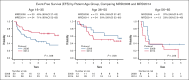
The enhanced utilization of native L-asparaginase (L-Asp) aims to improve treatment outcomes for adult patients with non-Philadelphia chromosome (Ph) acute lymphoblastic leukemia (ALL). In this measurable residual disease 2014 (MRD2014) study, we modified our protocol to include an augmented dose of native L-Asp. Compared with former MRD2008, the total dose of L-Asp was raised from 36 000 U/m2 to 232 000 U/m2 in patients aged 16 to 35 and from 36 000 U/m2 to 132 000 U/m2 in patients aged 36 to 65 years. Adult patients with ALL were enrolled between January 2014 and December 2019 based on the following eligibility criteria: non-L3 ALL, age 16 to 65 years, Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate liver and kidney functions (serum bilirubin ≤ 2.0 mg/dL; serum creatinine ≤ 2.0 mg/dL). The median follow-up time was 1128 days (range, 35-2400). A total of 81 patients with non-Ph ALL (40 males and 41 females; median age, 39 years [range, 16-64]) in whom MRD status was assessed were included. Complete remission was achieved in 72 patients (89%). The probability of 3-year event-free survival (EFS) and overall survival (OS) in these patients were 55% and 72%, respectively. The outcomes for patients aged 16 to 35 years demonstrated remarkable improvement. The 3-year EFS of MRD2008 at 45% significantly increased to 71% for MRD2014. Our study unequivocally demonstrated the beneficial effects of augmented use of L-Asp in this adolescent and young adult population. This trial was registered at UMIN Clinical Trials Registry as #UMIN000012382.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: K.N. has received honoraria/fees from Kyowa Kirin Co, Chugai Pharmaceutical Co, and Asahi Kasei Pharma Co. T.M. has received honoraria/fees from Takeda Pharmaceutical Co, Otsuka Pharmaceutical Co, MSD K.K., Astellas Pharma Inc, Janssen Pharmaceutical K.K., AbbVie Inc, and Kyowa Kirin Co; and research funding from Kyowa Kirin Co and Chugai Pharmaceutical Co. K. Kato has received honoraria/fees from Novartis Pharmaceuticals, Chugai Pharmaceutical Co, and Meiji Seika Pharma Co; and research funding from Chugai Pharmaceutical Co, Takeda Pharmaceutical Co, Kyowa Kirin Co, AbbVie Inc, Novartis Pharmaceuticals, Eisai Co Ltd, Janssen Pharmaceutical K.K., Ono Pharmaceutical Co, Meiji Seika Pharma Co, Daiichi Sankyo, MSD K.K., Bristol Myers Squibb Co, Gilead Sciences Inc, and Astellas Pharma Inc. T. Kamimura has received honoraria/fees from Janssen Pharmaceutical K.K., Ono Pharmaceutical Co, and AbbVie Inc. K.A. has received honoraria/fees from Asahi Kasei Pharma Co, Astellas Pharma Inc, AstraZeneca K.K., AbbVie Inc, Kyowa Kirin Co, Chugai Pharmaceutical Co, Bristol Myers Squibb Co, and Janssen Pharmaceutical K.K.; research funding from AbbVie Inc and Kyowa Kirin Co; and scholarship endowments/academic research funding from Otsuka Pharmaceutical Co, Nippon Shinyaku Co Ltd, Taiho Pharmaceutical Co Ltd, Asahi Kasei Pharma Co, Kyowa Kirin Co, Chugai Pharmaceutical Co, Sumitomo Pharmaceutical Co Ltd, AbbVie Inc, Eisai Co Ltd, and Takeda Pharmaceutical Co. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Different corticosteroids and regimens for accelerating fetal lung maturation for babies at risk of preterm birth.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD006764. doi: 10.1002/14651858.CD006764.pub4. Cochrane Database Syst Rev. 2022. PMID: 35943347 Free PMC article.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
References
-
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–543. - PubMed
-
- Brisco J, Hughes E, Neoh SH, et al. Relationship between minimal residual disease and outcome in adult acute lymphoblastic leukemia. Blood. 1996;87(12):5251–5256. - PubMed
-
- Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116–1123. - PubMed
LinkOut - more resources
Full Text Sources

