Efficacy of P62-expressing plasmid in treatment of canine osteoarthritis pain: a pilot study
- PMID: 40548246
- PMCID: PMC12179788
- DOI: 10.3389/fvets.2025.1519881
Efficacy of P62-expressing plasmid in treatment of canine osteoarthritis pain: a pilot study
Abstract
Introduction: Osteoarthritis (OA) is a progressive degenerative disease of synovial joints which is highly prevalent in dogs and results in lameness, loss of joint function and mobility, chronic pain, and reduced quality of life. Traditional OA management consist of non-steroidal anti-inflammatory drugs and remains challenging because of significant side effects, thus there is an urgent need for new effective and safe therapeutics for OA.
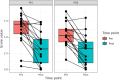
Methods: Here we present the results of our one-arm open-label pilot clinical study of our novel biologics, a DNA plasmid encoding SQSTM/p62, in 17 companion dogs suffering from OA. The dogs were injected intramuscularly with p62-plasmid once a week for 10 weeks, and pain relief was measured by owners weekly before injections using the CBPI (canine brief pain inventory) validated scale. The 11 parameters of CBPI are grouped in three major domains: pain severity score (PSS), pain interference score (PIS) and overall impression of the quality of life (QoL).
Results: Treatment with the p62-plasmid improved all 11 parameters of CBPI including PSS, PIS and QoL. Improvement in CBPI was observed after 2-4 weeks of treatment, whereas after 5-6 weeks of the treatment the parameters reached the plateau. After 10 weeks mean PSS score after the treatment decreased from 5.25 to 3.25, PIS score - from 7.0 to 3.27, and number of dogs with excellent and good QoL due to treatment increased from 1 to 12. Overall, the treatment success rate (i.e., a reduction ≥1 in PSS and ≥ 2 in PIS) was 90%. Importantly, no significant side effects of the p62-plasmid during the whole treatment period were observed.
Discussion: In this pilot study Elenagen demonstrated efficacy in treatment of OA pain in dogs without side effects. The study has some limitations: small animal number, lack of long-term follow-up, and the outcome is limited to only one parameter, CBPI. Also in future studies the mechanism of anti-OA effect of p62 plasmid should be addressed.
Keywords: DNA plasmid; chronic pain; inflammation; pilot trial; safety.
Copyright © 2025 Gabai, Bakin, Langs, Devlin, Krasny, Baranou, Polyakov, Patapovich, Gvozdev, Kardash, Bazyleuski, Yeliseyeu, Lelikov, Borodko and Shneider.
Conflict of interest statement
VG, RD, and AS were employed by CureLab Veterinary Inc. SG was employed by BELVITUNIFARM. EB was employed by CytoReason LTD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Update of
-
Efficacy of p62-expressing plasmid in treatment of canine osteoarthritis.Res Sq [Preprint]. 2024 Nov 21:rs.3.rs-5461004. doi: 10.21203/rs.3.rs-5461004/v1. Res Sq. 2024. Update in: Front Vet Sci. 2025 Jun 06;12:1519881. doi: 10.3389/fvets.2025.1519881. PMID: 39606451 Free PMC article. Updated. Preprint.
References
LinkOut - more resources
Full Text Sources

