Pig bile powder maintains blood glucose homeostasis by promoting glucagon-like peptide-1 secretion via inhibiting farnesoid X receptor
- PMID: 40548276
- PMCID: PMC12179914
- DOI: 10.4239/wjd.v16.i6.103616
Pig bile powder maintains blood glucose homeostasis by promoting glucagon-like peptide-1 secretion via inhibiting farnesoid X receptor
Abstract
Background: Traditional Chinese medicine offers many valuable remedies for maintaining blood glucose homeostasis in patients with type 2 diabetes mellitus. Bile powder (BP) is a powdered form of bile derived from pigs. It has been used historically in various medicinal applications. Currently, the therapeutic potential of BP in regulating glucose homeostasis remains unclear. Bile acids (BAs) are increasingly recognized for their role in glucose metabolism particularly through the modulation of glucagon-like peptide-1 (GLP-1).
Aim: To investigate BP effects on glucose homeostasis and elucidate its mechanistic role through GLP-1 and farnesoid X receptor (FXR) signaling.
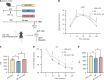
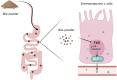
Methods: A diabetic mouse model was established using a high-fat diet and streptozotocin administration. Mice were treated with BP at doses of 25, 50, or 75 mg/kg/day for 45 days. Glucose homeostasis was assessed via the oral glucose tolerance test and insulin tolerance test. Serum GLP-1 levels were measured by enzyme-linked immunosorbent assay. A GLP-1 receptor antagonist and an FXR agonist were used to clarify the underlying mechanisms. In vitro STC-1 murine enteroendocrine cells were treated with a BP-mimicking BA mixture to assess GLP-1 secretion and proglucagon gene expression.
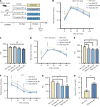
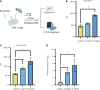
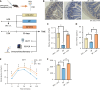
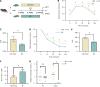
Results: BP treatment significantly improved glucose homeostasis in the diabetic mouse model as indicated by lower blood glucose (P < 0.05) and improved insulin sensitivity. BP enhanced GLP-1 secretion (P < 0.05), which was an effect abolished by the GLP-1 receptor antagonist. This observation confirmed its dependence on GLP-1 signaling. In STC-1 cells, BP-derived BA mixtures stimulated GLP-1 secretion and upregulated proglucagon expression (P < 0.05). Mechanistically, BP inhibited FXR signaling as evidenced by the reversal of its effects upon fexaramine administration. In addition, long-term BP treatment suppressed FXR signaling, resulting in elevated GLP-1 levels and preventing glucose dysregulation.
Conclusion: BP improved glucose homeostasis by promoting GLP-1 secretion via FXR inhibition, highlighting its potential as a therapeutic strategy for metabolic disorders.
Keywords: Farnesoid X receptor; Glucagon-like peptide 1; Pig bile powder; Traditional Chinese medicine; Type 2 diabetes mellitus.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Study on the modulation of kidney and liver function of rats with diabetic nephropathy by Huidouba through metabolomics.J Ethnopharmacol. 2025 Jul 24;351:120136. doi: 10.1016/j.jep.2025.120136. Epub 2025 Jun 11. J Ethnopharmacol. 2025. PMID: 40513925
-
Caffeoylquinic acids from Silphium perfoliatum L. show hepatoprotective effects on cholestatic mice by regulating enterohepatic circulation of bile acids.J Ethnopharmacol. 2025 Jan 30;337(Pt 2):118870. doi: 10.1016/j.jep.2024.118870. Epub 2024 Sep 30. J Ethnopharmacol. 2025. PMID: 39357582
-
Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus.Cochrane Database Syst Rev. 2017 May 10;5(5):CD012204. doi: 10.1002/14651858.CD012204.pub2. Cochrane Database Syst Rev. 2017. PMID: 28489279 Free PMC article.
-
Selective release of gastrointestinal hormones induced by an orally active GPR39 agonist.Mol Metab. 2021 Jul;49:101207. doi: 10.1016/j.molmet.2021.101207. Epub 2021 Mar 9. Mol Metab. 2021. PMID: 33711555 Free PMC article.
-
Insulin and glucose-lowering agents for treating people with diabetes and chronic kidney disease.Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011798. doi: 10.1002/14651858.CD011798.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246878 Free PMC article.
References
-
- He J, Lv L, Wang Z, Huo C, Zheng Z, Yin B, Jiang P, Yang Y, Li J, Gao Y, Xue J. Pulvis Fellis Suis extract attenuates ovalbumin-induced airway inflammation in murine model of asthma. J Ethnopharmacol. 2017;207:34–41. - PubMed
-
- He J, Liang J, Zhu S, Li J, Zhang Y, Sun W. Anti-inflammatory effects of Pulvis Fellis Suis extract in mice with ulcerative colitis. J Ethnopharmacol. 2011;138:53–59. - PubMed
-
- Qiao X, Ye M, Pan DL, Miao WJ, Xiang C, Han J, Guo DA. Differentiation of various traditional Chinese medicines derived from animal bile and gallstone: simultaneous determination of bile acids by liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr A. 2011;1218:107–117. - PubMed
-
- Kuang J, Wang J, Li Y, Li M, Zhao M, Ge K, Zheng D, Cheung KCP, Liao B, Wang S, Chen T, Zhang Y, Wang C, Ji G, Chen P, Zhou H, Xie C, Zhao A, Jia W, Zheng X, Jia W. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023;35:1752–1766.e8. - PubMed
-
- Zheng X, Chen T, Jiang R, Zhao A, Wu Q, Kuang J, Sun D, Ren Z, Li M, Zhao M, Wang S, Bao Y, Li H, Hu C, Dong B, Li D, Wu J, Xia J, Wang X, Lan K, Rajani C, Xie G, Lu A, Jia W, Jiang C, Jia W. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021;33:791–803.e7. - PubMed
LinkOut - more resources
Full Text Sources

