Lipidomic profiling of human adiposomes identifies specific lipid shifts linked to obesity and cardiometabolic risk
- PMID: 40548377
- PMCID: PMC12226050
- DOI: 10.1172/jci.insight.191872
Lipidomic profiling of human adiposomes identifies specific lipid shifts linked to obesity and cardiometabolic risk
Abstract
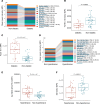
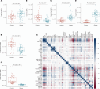
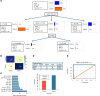
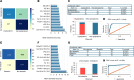
BACKGROUNDObesity, a growing health concern, often leads to metabolic disturbances, systemic inflammation, and vascular dysfunction. Emerging evidence suggests that adipose tissue-derived extracellular vesicles (adiposomes) may propagate obesity-related complications. However, their lipid composition and effect on cardiometabolic state remain unclear.METHODSThis study examined the lipid composition of adiposomes in 122 participants (75 in obesity group, 47 in lean group) and its connection to cardiometabolic risk. Adiposomes were isolated via ultracentrifugation and characterized using nanoparticle tracking and comprehensive lipidomic analysis by mass spectrometry. Cardiometabolic assessments included anthropometry, body composition, glucose-insulin homeostasis, lipid profiles, inflammatory markers, and vascular function.RESULTSCompared with lean controls, individuals with obesity exhibited elevated adiposome release and shifts in lipid composition, including higher ceramides, free fatty acids, and acylcarnitines, along with reduced levels of phospholipids and sphingomyelins. These alterations strongly correlated with increased BMI, insulin resistance, systemic inflammation, and impaired vascular function. Pathway enrichment analyses highlight dysregulation in glycerophospholipid and sphingolipid metabolism, bile secretion, proinflammatory pathways, and vascular contractility. Machine-learning models utilizing adiposome lipid data accurately classified obesity and predicted cardiometabolic conditions, such as diabetes, hypertension, dyslipidemia, and liver steatosis, achieving accuracy above 85%.CONCLUSIONObesity profoundly remodels the adiposome lipid landscape, linking lipid changes to inflammation, metabolic dysfunction, and vascular impairment. These findings underscore adiposome lipids as biomarkers for obesity and related cardiometabolic disorders, supporting personalized interventions and offering therapeutic value in risk stratification and treatment.FUNDINGThis project was supported by NIH grants R01HL161386, R00HL140049, P30DK020595 (PI: AMM), R01DK104927, and P30DK020595 as well as by a VA Merit Award (1I01BX003382, PI: BTL).
Keywords: Adipose tissue; Inflammation; Lipidomics; Metabolism; Obesity; Vascular biology.
Conflict of interest statement
Figures











Similar articles
-
Comparison of Adiposomal Lipids between Obese and Non-Obese Individuals.Metabolites. 2024 Aug 21;14(8):464. doi: 10.3390/metabo14080464. Metabolites. 2024. PMID: 39195560 Free PMC article.
-
The Primacy of Adipose Tissue Gene Expression and Plasma Lipidome in Cardiometabolic Disease in Persons With HIV.J Infect Dis. 2025 Feb 20;231(2):e407-e418. doi: 10.1093/infdis/jiae532. J Infect Dis. 2025. PMID: 39657693 Free PMC article.
-
Integrated plasma metabolomics and lipidomics profiling highlight distinctive signatures with hashimoto's thyroiditis.Sci Rep. 2025 Jul 1;15(1):21081. doi: 10.1038/s41598-025-07905-7. Sci Rep. 2025. PMID: 40595156 Free PMC article.
-
Gut microbiome-based interventions for the management of obesity in children and adolescents aged up to 19 years.Cochrane Database Syst Rev. 2025 Jul 10;7(7):CD015875. doi: 10.1002/14651858.CD015875. Cochrane Database Syst Rev. 2025. PMID: 40637175 Review.
-
Unlocking the secrets: Adipose tissue dysfunction and atherosclerosis-mechanisms and innovative therapeutic approaches.Atherosclerosis. 2025 Sep;408:120424. doi: 10.1016/j.atherosclerosis.2025.120424. Epub 2025 Jul 10. Atherosclerosis. 2025. PMID: 40683112 Review.
Cited by
-
Circulating Lipid Profiles Indicate Incomplete Metabolic Recovery After Weight Loss, Suggesting the Need for Additional Interventions in Severe Obesity.Biomolecules. 2025 Aug 1;15(8):1112. doi: 10.3390/biom15081112. Biomolecules. 2025. PMID: 40867557 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

