ER Stress Induced by Artemisinin and Its Derivatives Determines the Susceptibility to Their Synergistic Apoptotic Killing With TRAIL
- PMID: 40548420
- PMCID: PMC12183610
- DOI: 10.1002/cam4.71001
ER Stress Induced by Artemisinin and Its Derivatives Determines the Susceptibility to Their Synergistic Apoptotic Killing With TRAIL
Abstract
Aim: Artemisinins are a class of antimalarial drugs that are lately being researched for their antitumor activity. We previously reported that artesunate, an artemisinin derivative, can induce ferroptosis and enhance TRAIL (Tumor necrosis factor-Related Apoptosis-Inducing Ligand)-induced apoptosis. Here we investigated the role of endoplasmic reticulum (ER) stress induced by artemisinin and its derivatives, especially in the enhancement of TRAIL-induced apoptosis, which can be exploited for repurposing the use of artemisinins in cancer therapy.
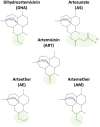
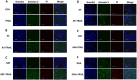
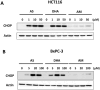
Methods: We show in this study a comparative profile of the ER stress induced by different derivatives of this drug, namely artemisinin, artesunate, arteether, artemether, and dihydroartemisinin, all of which are available readily and approved for treating human patients. These five derivatives were used to treat human colon carcinoma HCT116 cells and pancreatic adenocarcinoma BxPC3 cells over a range of doses.
Results: Our data show a highly significant positive correlation between ER stress caused by these drugs and their corresponding apoptotic susceptibilities upon treatment with TRAIL.
Conclusion: We concluded that dihydroartemisinin is the most effective contender among all the derivatives tested to enhance TRAIL-induced apoptosis.
Keywords: ER stress; apoptosis; artemisinins; cytotoxicity; ferroptosis.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Mcl-1 is a Gatekeeper Molecule to Regulate the Crosstalk Between Ferroptotic Agent-Induced ER Stress and TRAIL-Induced Apoptosis.J Cell Biochem. 2025 Jan;126(1):e30681. doi: 10.1002/jcb.30681. J Cell Biochem. 2025. PMID: 39853862 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Surveillance for Violent Deaths - National Violent Death Reporting System, 50 States, the District of Columbia, and Puerto Rico, 2022.MMWR Surveill Summ. 2025 Jun 12;74(5):1-42. doi: 10.15585/mmwr.ss7405a1. MMWR Surveill Summ. 2025. PMID: 40493548 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
References
-
- Yang W. S., Kim K. J., Gaschler M. M., Patel M., Shchepinov M. S., and Stockwell B. R., “Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis,” Proceedings of the National Academy of Sciences of the United States of America 113 (2016): E4966–E4975, 10.1073/pnas.1603244113. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

