PDZ-directed substrate recruitment is the primary determinant of specific 4E-BP1 dephosphorylation by PP1-Neurabin
- PMID: 40548551
- PMCID: PMC12185105
- DOI: 10.7554/eLife.103403
PDZ-directed substrate recruitment is the primary determinant of specific 4E-BP1 dephosphorylation by PP1-Neurabin
Abstract
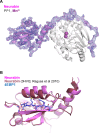
Phosphoprotein phosphatase 1 (PP1) relies on association with PP1-interacting proteins (PIPs) to generate substrate-specific PIP/PP1 holoenzymes, but the lack of well-defined substrates has hindered elucidation of the mechanisms involved. We previously demonstrated that the Phactr1 PIP confers sequence specificity on the Phactr1/PP1 holoenzyme by remodelling the PP1 hydrophobic substrate groove. Phactr1 defines a group of 'RVxF-ΦΦ-R-W' PIPs that all interact with PP1 in a similar fashion. Here, we use a PP1-PIP fusion approach to address sequence specificity and identify substrates of the RVxF-ΦΦ-R-W family PIPs. We show that the four Phactr proteins confer identical sequence specificities on their holoenzymes. We identify the 4E-BP and p70 S6K translational regulators as substrates for the Neurabin/Spinophilin PIPs, implicated in neuronal plasticity, pointing to a role for their holoenzymes in mTORC1-dependent translational control. Biochemical and structural experiments show that in contrast to the Phactrs, substrate recruitment and catalytic efficiency of the PP1-Neurabin and PP1-Spinophilin fusions is primarily determined by substrate interaction with the PDZ domain adjoining their RVxF-ΦΦ-R-W motifs, rather than by recognition of the remodelled PP1 hydrophobic groove. Thus, even PIPs that interact with PP1 in a similar manner use different mechanisms to ensure substrate selectivity.
Keywords: 4E-BP1; Neurabin; PIP; Phactr; biochemistry; chemical biology; human; mTORC1; molecular biophysics; phosphatase; structural biology.
© 2024, Fedoryshchak et al.
Conflict of interest statement
RF, KE, DJ, SM, RT No competing interests declared
Figures












Update of
- doi: 10.1101/2024.09.23.614477
- doi: 10.7554/eLife.103403.1
- doi: 10.7554/eLife.103403.2
Similar articles
-
A protein phosphatase 1 specific phosphatase targeting peptide (PhosTAP) to identify the PP1 phosphatome.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2415383121. doi: 10.1073/pnas.2415383121. Epub 2024 Oct 24. Proc Natl Acad Sci U S A. 2024. PMID: 39446389 Free PMC article.
-
Molecular basis for substrate specificity of the Phactr1/PP1 phosphatase holoenzyme.Elife. 2020 Sep 25;9:e61509. doi: 10.7554/eLife.61509. Elife. 2020. PMID: 32975518 Free PMC article.
-
LIMK2-1 Is a Phosphorylation-Dependent Inhibitor of Protein Phosphatase-1 Catalytic Subunit and Myosin Phosphatase Holoenzyme.Int J Mol Sci. 2025 Jul 30;26(15):7347. doi: 10.3390/ijms26157347. Int J Mol Sci. 2025. PMID: 40806479 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
References
-
- Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, Ronneberger O, Willmore L, Ballard AJ, Bambrick J, Bodenstein SW, Evans DA, Hung C-C, O’Neill M, Reiman D, Tunyasuvunakool K, Wu Z, Žemgulytė A, Arvaniti E, Beattie C, Bertolli O, Bridgland A, Cherepanov A, Congreve M, Cowen-Rivers AI, Cowie A, Figurnov M, Fuchs FB, Gladman H, Jain R, Khan YA, Low CMR, Perlin K, Potapenko A, Savy P, Singh S, Stecula A, Thillaisundaram A, Tong C, Yakneen S, Zhong ED, Zielinski M, Žídek A, Bapst V, Kohli P, Jaderberg M, Hassabis D, Jumper JM. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500. doi: 10.1038/s41586-024-07487-w. - DOI - PMC - PubMed
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica. Section D, Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Aguilar-Valles A, Matta-Camacho E, Khoutorsky A, Gkogkas C, Nader K, Lacaille JC, Sonenberg N. Inhibition of group I metabotropic glutamate receptors reverses autistic-like phenotypes caused by deficiency of the translation repressor eIF4E binding protein 2. The Journal of Neuroscience. 2015;35:11125–11132. doi: 10.1523/JNEUROSCI.4615-14.2015. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

